Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma
et al., European Respiratory Journal, doi:10.1183/13993003.01724-2021, DAWn-plasma, NCT04429854, Aug 2021
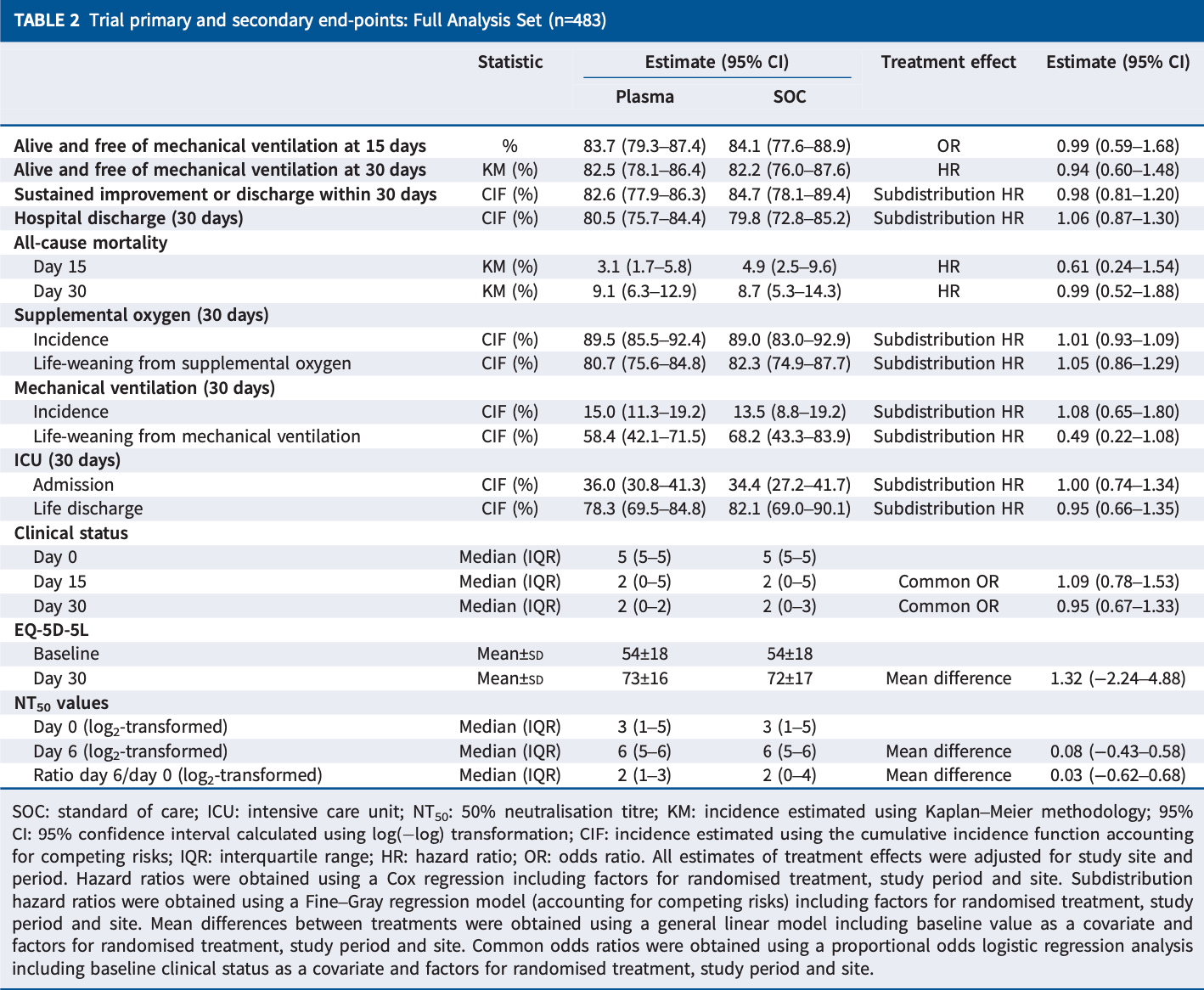
RCT 489 hospitalized COVID-19 patients in Belgium, showing no significant difference in outcomes with convalescent plasma.
|
risk of death, 1.0% lower, HR 0.99, p = 0.98, treatment 320, control 163.
|
|
risk of mechanical ventilation, 8.0% higher, HR 1.08, p = 0.78, treatment 320, control 163.
|
|
risk of ICU admission, no change, HR 1.00, p = 1.00, treatment 320, control 163.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Devos et al., 26 Aug 2021, Randomized Controlled Trial, Belgium, peer-reviewed, 26 authors, study period 2 May, 2020 - 26 January, 2021, average treatment delay 7.0 days, trial NCT04429854 (history) (DAWn-plasma).
Contact: geert.meyfroidt@uzleuven.be.
Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma
European Respiratory Journal, doi:10.1183/13993003.01724-2021
Early transfusion of 4 units of high neutralising antibody titre convalescent plasma in hospitalised COVID-19 patients does not reduce mortality or the need for mechanical ventilation https://bit.ly/ 3fiRY2I
References
Abani, Abbas, Abbas, Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial, Lancet
Agarwal, Mukherjee, Kumar, Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -final report, N Engl J Med
Betrains, Godinas, Woei-A-Jin, Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies, Br J Haematol
Devos, Geukens, Schauwvlieghe, A randomized, multicentre, open-label phase II proof-of-concept trial investigating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19: the Donated Antibodies Working against nCoV (DAWn-Plasma) trial, Trials
Gharbharan, Jordans, Geurtsvankessel, Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection, Nat Commun
Goldberg, Zvi, Sheena, A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel, Clin Microbiol Infect
Grasselli, Pesenti, Cecconi, Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response, JAMA
Guimarães, Quirk, Furtado, Tofacitinib in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Hueso, Pouderoux, Péré, Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19, Blood
Janiaud, Axfors, Schmitt, Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis, JAMA
Joyner, Carter, Senefeld, Convalescent plasma antibody levels and the risk of death from Covid-19, N Engl J Med
Libster, Marc, Wappner, Early high-titer plasma therapy to prevent severe Covid-19 in older adults, N Engl J Med
O'donnell, Grinsztejn, Cummings, A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19, J Clin Invest
Rubin, Multiple imputation after 18+ years, J Am Stat Assoc
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med
Simonovich, Pratx, Scibona, A randomized trial of convalescent plasma in Covid-19 severe pneumonia, N Engl J Med
Taccone, Van Goethem, Pauw, The role of organizational characteristics on the outcome of COVID-19 patients admitted to the ICU in Belgium, Lancet Reg Health Eur
The, Group, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med
The, Investigators, Interleukin-6 receptor antagonists in critically ill patients with Covid-19, N Engl J Med
Verity, Okell, Dorigatti, Estimates of the severity of coronavirus disease 2019: a model-based analysis, Lancet Infect Dis
DOI record:
{
"DOI": "10.1183/13993003.01724-2021",
"ISSN": [
"0903-1936",
"1399-3003"
],
"URL": "http://dx.doi.org/10.1183/13993003.01724-2021",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>Several randomised clinical trials have studied convalescent plasma for coronavirus disease 2019 (COVID-19) using different protocols, with different severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) neutralising antibody titres, at different time-points and severities of illness.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>In the prospective multicentre DAWn-plasma trial, adult patients hospitalised with COVID-19 were randomised to 4 units of open-label convalescent plasma combined with standard of care (intervention group) or standard of care alone (control group). Plasma from donors with neutralising antibody titres (50% neutralisation titre (NT<jats:sub>50</jats:sub>)) ≥1/320 was the product of choice for the study.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>Between 2 May 2020 and 26 January 2021, 320 patients were randomised to convalescent plasma and 163 patients to the control group according to a 2:1 allocation scheme. A median (interquartile range) volume of 884 (806–906) mL) convalescent plasma was administered and 80.68% of the units came from donors with neutralising antibody titres (NT<jats:sub>50</jats:sub>) ≥1/320. Median time from onset of symptoms to randomisation was 7 days. The proportion of patients alive and free of mechanical ventilation on day 15 was not different between both groups (convalescent plasma 83.74% (n=267) <jats:italic>versus</jats:italic> control 84.05% (n=137)) (OR 0.99, 95% CI 0.59–1.66; p=0.9772). The intervention did not change the natural course of antibody titres. The number of serious or severe adverse events was similar in both study arms and transfusion-related side-effects were reported in 19 out of 320 patients in the intervention group (5.94%).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Transfusion of 4 units of convalescent plasma with high neutralising antibody titres early in hospitalised COVID-19 patients did not result in a significant improvement of clinical status or reduced mortality.</jats:p></jats:sec>",
"alternative-id": [
"10.1183/13993003.01724-2021"
],
"author": [
{
"affiliation": [],
"family": "Devos",
"given": "Timothy",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-3260-280X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Van Thillo",
"given": "Quentin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Compernolle",
"given": "Veerle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Najdovski",
"given": "Tomé",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romano",
"given": "Marta",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7697-6849",
"affiliation": [],
"authenticated-orcid": false,
"family": "Dauby",
"given": "Nicolas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jadot",
"given": "Laurent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leys",
"given": "Mathias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maillart",
"given": "Evelyne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Loof",
"given": "Sarah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Seyler",
"given": "Lucie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moonen",
"given": "Martial",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moutschen",
"given": "Michel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Van Regenmortel",
"given": "Niels",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ariën",
"given": "Kevin K.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barbezange",
"given": "Cyril",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Betrains",
"given": "Albrecht",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Garigliany",
"given": "Mutien",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Engelen",
"given": "Matthias M.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4068-7228",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gyselinck",
"given": "Iwein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maes",
"given": "Piet",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schauwvlieghe",
"given": "Alexander",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5616-8548",
"affiliation": [],
"authenticated-orcid": false,
"family": "Liesenborghs",
"given": "Laurens",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Belmans",
"given": "Ann",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8698-2858",
"affiliation": [],
"authenticated-orcid": false,
"family": "Verhamme",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meyfroidt",
"given": "Geert",
"sequence": "additional"
}
],
"container-title": "European Respiratory Journal",
"container-title-short": "Eur Respir J",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"ersjournals.com"
]
},
"created": {
"date-parts": [
[
2021,
8,
26
]
],
"date-time": "2021-08-26T16:16:09Z",
"timestamp": 1629994569000
},
"deposited": {
"date-parts": [
[
2022,
2,
10
]
],
"date-time": "2022-02-10T09:30:34Z",
"timestamp": 1644485434000
},
"funder": [
{
"DOI": "10.13039/501100003130",
"award": [
"1843118N"
],
"doi-asserted-by": "publisher",
"name": "Fonds Wetenschappelijk Onderzoek"
},
{
"DOI": "10.13039/501100019353",
"doi-asserted-by": "crossref",
"name": "Belgian Health Care Knowledge Centre"
}
],
"indexed": {
"date-parts": [
[
2022,
12,
17
]
],
"date-time": "2022-12-17T10:50:25Z",
"timestamp": 1671274225430
},
"is-referenced-by-count": 25,
"issue": "2",
"issued": {
"date-parts": [
[
2021,
8,
26
]
]
},
"journal-issue": {
"issue": "2",
"published-online": {
"date-parts": [
[
2022,
2,
10
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
26
]
],
"date-time": "2021-08-26T00:00:00Z",
"timestamp": 1629936000000
}
}
],
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1183/13993003.01724-2021",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "81",
"original-title": [],
"page": "2101724",
"prefix": "10.1183",
"published": {
"date-parts": [
[
2021,
8,
26
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
26
]
]
},
"published-print": {
"date-parts": [
[
2022,
2
]
]
},
"publisher": "European Respiratory Society (ERS)",
"reference": [
{
"key": "2022021001300748000_59.2.2101724.1",
"unstructured": "World Health Organization . WHO coronavirus (COVID-19) dashboard with vaccination data. 2021. https://covid19.who.int Date last accessed: 16 July 2021."
},
{
"DOI": "10.1001/jama.2020.4031",
"article-title": "Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response",
"author": "Grasselli",
"doi-asserted-by": "crossref",
"first-page": "1545",
"journal-title": "JAMA",
"key": "2022021001300748000_59.2.2101724.2",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30243-7",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.3"
},
{
"DOI": "10.1016/j.lanepe.2020.100019",
"article-title": "The role of organizational characteristics on the outcome of COVID-19 patients admitted to the ICU in Belgium",
"author": "Taccone",
"doi-asserted-by": "crossref",
"first-page": "100019",
"journal-title": "Lancet Reg Health Eur",
"key": "2022021001300748000_59.2.2101724.4",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2021436",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.5"
},
{
"article-title": "Interleukin-6 receptor antagonists in critically ill patients with Covid-19",
"first-page": "1491",
"journal-title": "N Engl J Med",
"key": "2022021001300748000_59.2.2101724.6",
"volume": "84",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2030340",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.7"
},
{
"DOI": "10.1056/nejmoa2101643",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.8"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.9"
},
{
"DOI": "10.1056/NEJMoa2023184",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.10"
},
{
"DOI": "10.1016/j.cmi.2021.02.029",
"article-title": "A real-life setting evaluation of the effect of remdesivir on viral load in COVID-19 patients admitted to a large tertiary centre in Israel",
"author": "Goldberg",
"doi-asserted-by": "crossref",
"first-page": "917.e1",
"journal-title": "Clin Microbiol Infect",
"key": "2022021001300748000_59.2.2101724.11",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3939",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.12"
},
{
"DOI": "10.1056/NEJMoa2031304",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.13"
},
{
"DOI": "10.1038/s41467-021-23469-2",
"article-title": "Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection",
"author": "Gharbharan",
"doi-asserted-by": "crossref",
"first-page": "3189",
"journal-title": "Nat Commun",
"key": "2022021001300748000_59.2.2101724.14",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2033700",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.15"
},
{
"DOI": "10.1001/jama.2021.2747",
"article-title": "Association of convalescent plasma treatment with clinical outcomes in patients with COVID-19: a systematic review and meta-analysis",
"author": "Janiaud",
"doi-asserted-by": "crossref",
"first-page": "1185",
"journal-title": "JAMA",
"key": "2022021001300748000_59.2.2101724.16",
"volume": "325",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.17"
},
{
"DOI": "10.1186/s13063-020-04876-0",
"article-title": "A randomized, multicentre, open-label phase II proof-of-concept trial investigating the clinical efficacy and safety of the addition of convalescent plasma to the standard of care in patients hospitalized with COVID-19: the Donated Antibodies Working against nCoV (DAWn-Plasma) trial",
"author": "Devos",
"doi-asserted-by": "crossref",
"first-page": "981",
"journal-title": "Trials",
"key": "2022021001300748000_59.2.2101724.18",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.2307/2291635",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.19"
},
{
"DOI": "10.1016/s0140-6736(21)00897-7",
"doi-asserted-by": "publisher",
"key": "2022021001300748000_59.2.2101724.20"
},
{
"DOI": "10.1172/JCI150646",
"article-title": "A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19",
"author": "O'Donnell",
"doi-asserted-by": "crossref",
"first-page": "e150646",
"journal-title": "J Clin Invest",
"key": "2022021001300748000_59.2.2101724.21",
"volume": "131",
"year": "2021"
},
{
"DOI": "10.1111/bjh.17266",
"article-title": "Convalescent plasma treatment of persistent severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection in patients with lymphoma with impaired humoral immunity and lack of neutralising antibodies",
"author": "Betrains",
"doi-asserted-by": "crossref",
"first-page": "1100",
"journal-title": "Br J Haematol",
"key": "2022021001300748000_59.2.2101724.22",
"volume": "192",
"year": "2021"
},
{
"DOI": "10.1182/blood.2020008423",
"article-title": "Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19",
"author": "Hueso",
"doi-asserted-by": "crossref",
"first-page": "2290",
"journal-title": "Blood",
"key": "2022021001300748000_59.2.2101724.23",
"volume": "136",
"year": "2020"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "http://erj.ersjournals.com/lookup/doi/10.1183/13993003.01724-2021"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1183/ers-crossmark-policy",
"volume": "59"
}