Low dose of hydroxychloroquine is associated with reduced COVID-19 mortality: a multicenter study in China
et al., Frontiers of Medicine, doi:10.1007/s11684-025-1123-9, NCT05615792, Mar 2025
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
PSM retrospective 53,030 hospitalized patients in China showing low dose HCQ treatment associated with significantly lower all-cause mortality, mechanical ventilation, acute heart injury, and acute kidney injury, with benefits consistent across mild and critically ill patients.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
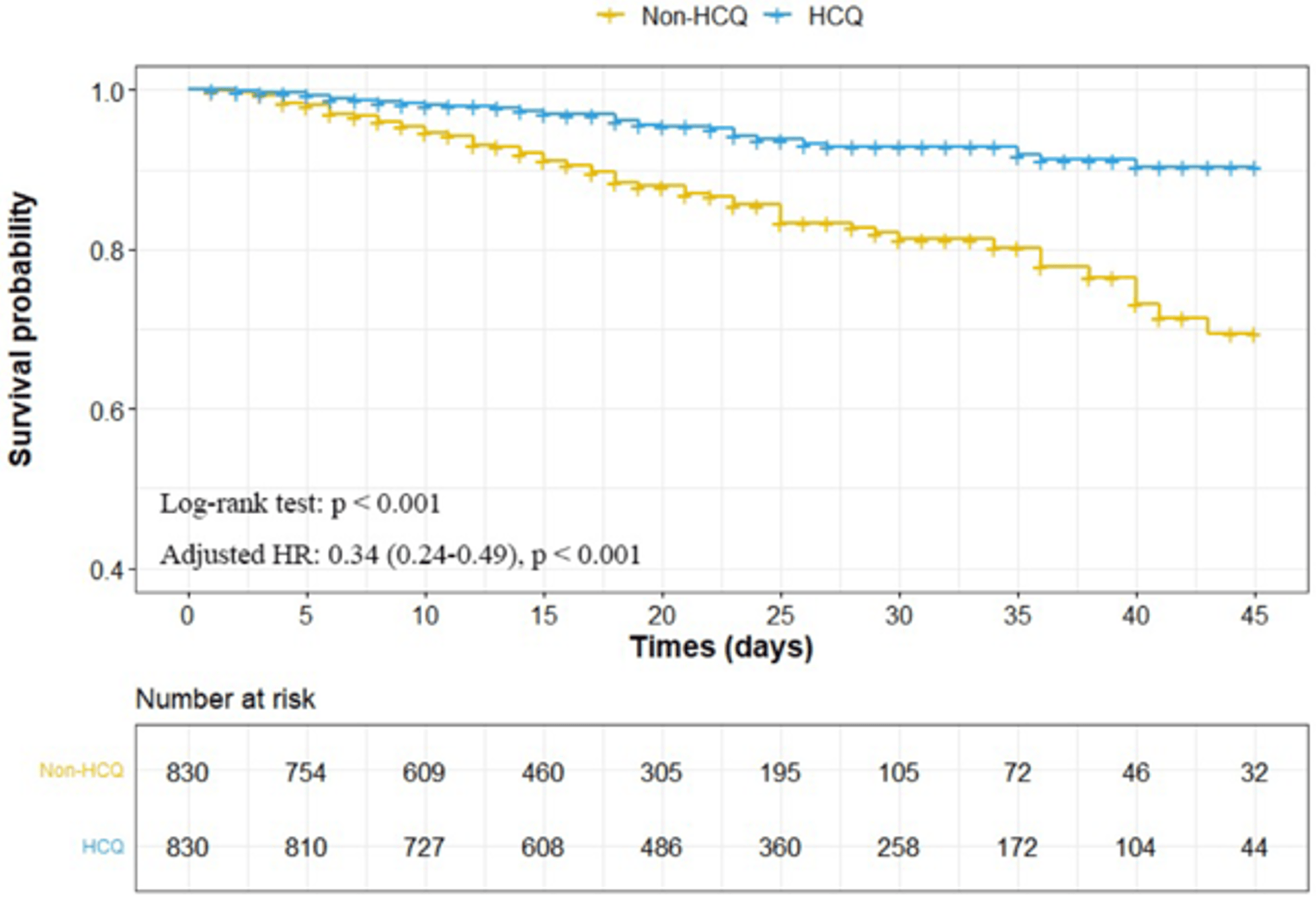
risk of death, 66.0% lower, HR 0.34, p < 0.001, treatment 830, control 830, all patients, propensity score matching, Kaplan-Meier.
|
|
risk of death, 74.0% lower, HR 0.26, p < 0.001, treatment 800, control 800, low dose, propensity score matching, Kaplan-Meier.
|
|
risk of mechanical ventilation, 24.8% lower, HR 0.75, p = 0.05, treatment 841, control 52,189, inverted to make HR<1 favor treatment, all patients, Kaplan-Meier.
|
|
risk of mechanical ventilation, 27.0% lower, HR 0.73, p = 0.04, treatment 800, control 52,189, low dose, Kaplan-Meier.
|
|
ARDS, 40.8% lower, HR 0.59, p = 0.21, treatment 841, control 52,189, inverted to make HR<1 favor treatment, all patients, Kaplan-Meier.
|
|
ARDS, 49.0% lower, HR 0.51, p = 0.13, treatment 800, control 52,189, low dose, Kaplan-Meier.
|
|
AKI, 31.0% lower, HR 0.69, p = 0.005, treatment 841, control 52,189, inverted to make HR<1 favor treatment, all patients, Kaplan-Meier.
|
|
AKI, 30.0% lower, HR 0.70, p = 0.008, treatment 800, control 52,189, low dose, Kaplan-Meier.
|
|
acute heart injury, 37.9% lower, HR 0.62, p = 0.03, treatment 841, control 52,189, inverted to make HR<1 favor treatment, all patients, Kaplan-Meier.
|
|
acute heart injury, 39.0% lower, HR 0.61, p = 0.02, treatment 800, control 52,189, low dose, Kaplan-Meier.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
He et al., 4 Mar 2025, retrospective, China, peer-reviewed, 9 authors, study period 29 December, 2019 - 31 August, 2021, trial NCT05615792 (history).
Contact: dwwang@tjh.tjmu.edu.cn.
Abstract: Front. Med.
https://doi.org/10.1007/s11684-025-1123-9
LETTER TO FRONTIERS OF MEDICINE
Low dose of hydroxychloroquine is associated with reduced
COVID-19 mortality: a multicenter study in China
✉)1
Wu He1,*, Ke Xu1,*, Yongcui Yan1, Gen Li1, Bo Yu1, Junfang Wu1, Kaineng Zhong2, Da Zhou2, Dao Wen Wang (
1Division of Cardiology, Department of Internal Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and
Technology; Hubei Key Laboratory of Genetics and Molecular Mechanisms of Cardiological Disorders, Wuhan 430030, China; 2Health
Commission of Hubei Province, Wuhan 430079, China
© Higher Education Press 2025
Dear Editor,
Since December 2019, severe acute respiratory
syndrome coronavirus 2 (SARS-CoV-2) has widely
spread worldwide, and we have been fighting against
coronavirus disease 2019 (COVID-19) for more than 4
years [1]. According to the statistics of the World Health
Organization (WHO), more than 775 million individuals
have incurred COVID-19, and 7 million deaths have been
recorded. The COVID-19 outbreak has become a
devastating global health crisis, and the challenges faced
by humanity due to this disease are continuing [2]. In the
past 4 years, an old antimalarial drug, hydroxychloroquine (HCQ), has been evaluated by doctors and
scientists [3]. Its effects on the immune system have been
fully confirmed, including inhibition of Toll-like receptor
signals and lymphocyte receptors; interference with
lysosomal acidification, antigen presentation, and DNA
binding and stabilization; and reduction of proinflammatory cytokines produced by macrophages
(especially IL-1, IL-6, and TNF-α) [4]. In vitro
experiment of HCQ showed its efficacy in inhibiting
novel coronaviruses and its greater effectiveness than
chloroquine [5]. Our previous clinical results also
supported that HCQ has good therapeutic effects on
patients with COVID-19 [6]. An observational study
revealed that the use of HCQ was associated with a
reduced hospitalization rate among patients with COVID19 [7]. Nevertheless, some studies reported neutral or
negative findings on the clinical results and meta-analysis
of patients with COVID-19 treated with HCQ [8], leading
to doubts and restrictions on the clinical use of HCQ on
patients with COVID-19 to a certain extent.
We conducted a multicenter retrospective study of
Received August 5, 2024; accepted December 16, 2024
Correspondence: Dao Wen Wang, dwwang@tjh.tjmu.edu.cn
*These authors contributed equally to this work.
53 030 patients with COVID-19 (discharged or deceased)
in 138 hospitals in Hubei Province to clarify the effects of
different HCQ doses on the mortality of patients with
COVID-19 (Trial registration: NCT05615792). Under
China and WHO interim guidance, all the patients
diagnosed with COVID-19 between Dec 29, 2019 (i.e.,
when the first patients were admitted) and Aug 31, 2021
were screened, and those who had died or were
discharged were included in this study. The patients were
divided into HCQ group and non-HCQ group according
to whether or not they were administered with HCQ. A
1:1 propensity score matching (PSM) analysis was used
to balance the confounding factors between HCQ group
and non-HCQ group. The HCQ group consisted of
patients with COVID-19 who continuously received HCQ
for more than 3 days. According to the dosage, the HCQ
group was subdivided into high-dose (≥ 400 mg/d) and
low-dose (< 400 mg/d) groups.
This study included 68 128 patients with COVID-19
from 138 hospitals..
