Effect of favipiravir use on INR, PT, aPTT tests of COVID-19 patients
et al., Sabuncuoglu Serefeddin Health Sciences, doi:10.55895/sshs.1213382, Jan 2023
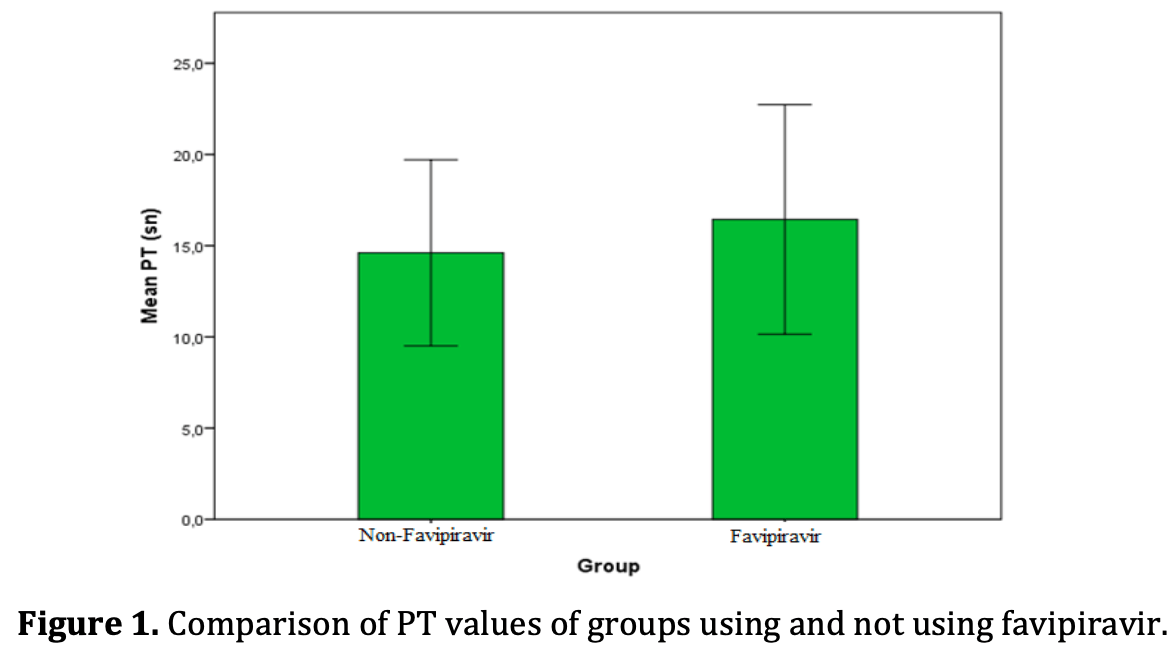
Retrospective 100 patients in Turkey analyzing the effects of favipiravir on coagulation parameters in COVID-19 patients. Results showed prothrombin time (PT) and international normalized ratio (INR) were significantly prolonged in the favipiravir group, indicating impaired clotting ability. However, no difference was seen in activated partial thromboplastin time (aPTT) between groups. The authors conclude favipiravir prolongs clotting time, which could increase bleeding risk. They recommend close monitoring of coagulation and potential dosage adjustments of anticoagulants in COVID-19 patients receiving favipiravir. Further research is needed to confirm the coagulation effects of this antiviral medication.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Gül et al., 3 Jan 2023, retrospective, Turkey, peer-reviewed, 4 authors, study period June 2021 - March 2022.
Contact: mehmetali.gul@amasya.edu.tr.
EFFECT OF FAVIPIRAVIR USE ON INR, PT, APTT TESTS OF COVID-19 PATIENTS
The 2019 coronavirus disease (COVID-19) has caused millions of cases worldwide. As the pandemic progresses, understanding the effects of this disease remains important. We aimed to examine the hematological effects of the disease. The research was carried out as a retrospective study, 50 patients using favipiravir and 50 patients not using favipiravir who had positive COVID-19 RT-PCR test in nasal and throat swabs were included in the study. INR, PTT, aPTT tests were evaluated on all patients. Results of patients using favipiravir; INR 1.3±0.2, PT(s) 16.4±3.4, aPTT(s) 40.7±10.1, while the results of patients who did not use favipiravir were INR 1,2±0.2, PT(s) 14.6±2.5, aPTT(s) was found 38.4±7.8. While PT and INR were found to significantly higher in patients using favipiravir (p<0.05), the elevation in aPTT values was not significant. As a consequence, it was obtained that favipiravir prolongs the clotting time. In the light of these results, it is recommended to consider this in anticoagulant therapy used for treatment.
Information This study was presented as an oral presentation at the VII. Turkey In Vitro Diagnostics (IVD) Symposium 2022. We would like to thank the staff and doctors of Sabuncuoğlu Şerefeddin Training and Research Hospital (especially laboratories and clinics related to COVID-19), who worked with great dedication.
Conflicts of interest The authors declare that there are no potential conflicts of interest relevant to this article. Gul M. A., Kurt N., Capraz M., Ozturk A. (2022) . Effect of Favipiravir Use on INR, PT, aPTT Tests of 4(3), [14] [15] [16] [17] [18] [19] [20]
References
Akbari, Tabrizi, Lankarani, Aria, Vakili et al., The role of cytokine profile and lymphocyte subsets in the severity of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis, Life Sciences, doi:10.1016/j.lfs.2020.118167
Atçalı, Yakut, Çağlayan, Ulucan, Kara, Effects of favipiravir on hematologic parameters and bone marrow in the rats, Journal of Experimental and Clinical Medicine
Bert, Tan, Kunasegaran, Tham, Hafezi et al., SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls, Nature, doi:10.1038/s41586-020-2550-z
Ciaccio, Agnello, Biochemical biomarkers alterations in Coronavirus Disease 2019 (COVID-19), Diagnosis (Berl), doi:10.1515/dx-2020-0057
Connors, Levy, COVID-19 and its implications for thrombosis and anticoagulation, Blood, doi:10.1182/blood.2020006000
Conway, Mackman, Warren, Wolberg, Mosnier et al., Understanding COVID-19-associated coagulopathy, Nat Rev Immunol, doi:10.1038/s41577-022-00762-9
De Clercq, New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections, Chem Asian J, doi:10.1002/asia.201900841
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci, doi:10.2183/pjab.93.027
Gul, Kurt, Capraz, Ozturk, Effect of Favipiravir Use on INR, PT, aPTT Tests of COVID-19 Patients Sabuncuoglu, Serefeddin Health Science
Mir, Tahamtan, Nikoo, Arabi, Moradi et al., Evaluation of biochemical characteristics of 183 COVID-19 patients: A retrospective study, Gene Rep, doi:10.1016/j.genrep.2021.101448
Shang, Wan, Liu, Yount, Gully et al., Structure of mouse coronavirus spike protein complexed with receptor reveals mechanism for viral entry, PLoS Pathog, doi:10.1371/journal.ppat.1008392
Thachil, What do monitoring platelet counts in COVID-19 teach us, Journal of Thrombosis and Haemostasis, doi:10.1111/jth.14879
Wang, Zhong, Salam, Tarning, Zhan et al., Phase 2a, openlabel, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza, EBioMedicine, doi:10.1016/j.ebiom.2020.103125
Yang, Cao, Qin, Wang, Cheng et al., Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China, J Infect, doi:10.1016/j.jinf.2020.02.016
Yaylaci, Dheir, Senocak, Genc, Kocayigit et al., The effects of favipiravir on hematological parameters of covid-19 patients, Rev Assoc Med Bras, doi:10.1590/1806-9282.66.S2
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet, doi:10.1016/S0140-6736(20)30566-3
Zinellu, Paliogiannis, Carru, Mangoni, INR and COVID-19 severity and mortality: A systematic review with meta-analysis and meta-regression, Adv Med Sci, doi:10.1016/j.advms.2021.07.009
DOI record:
{
"DOI": "10.55895/sshs.1213382",
"ISSN": [
"2667-6338"
],
"URL": "http://dx.doi.org/10.55895/sshs.1213382",
"abstract": "<jats:p xml:lang=\"en\">The 2019 coronavirus disease (COVID-19) has caused millions of cases worldwide. As the pandemic progresses, understanding the effects of this disease remains important. We aimed to examine the hematological effects of the disease. The research was carried out as a retrospective study, 50 patients using favipiravir and 50 patients not using favipiravir who had positive COVID-19 RT-PCR test in nasal and throat swabs were included in the study. INR, PTT, aPTT tests were evaluated on all patients. Results of patients using favipiravir; INR 1.3±0.2, PT(s) 16.4±3.4, aPTT(s) 40.7±10.1, while the results of patients who did not use favipiravir were INR 1,2±0.2, PT(s) 14.6±2.5, aPTT(s) was found 38.4±7.8. While PT and INR were found to significantly higher in patients using favipiravir (p&lt;0.05), the elevation in aPTT values was not significant. As a consequence, it was obtained that favipiravir prolongs the clotting time. In the light of these results, it is recommended to consider this in anticoagulant therapy used for treatment.</jats:p>",
"accepted": {
"date-parts": [
[
2022,
12,
19
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-5849-0116",
"affiliation": [
{
"name": "AMASYA ÜNİVERSİTESİ, TIP FAKÜLTESİ"
}
],
"authenticated-orcid": true,
"family": "GÜL",
"given": "Mehmet Ali",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-1685-5332",
"affiliation": [
{
"name": "Erzincan Binali Yıldırım Üniversitesi Tıp Fakültesi"
}
],
"authenticated-orcid": true,
"family": "KURT",
"given": "Nezahat",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9586-6509",
"affiliation": [
{
"name": "AMASYA ÜNİVERSİTESİ, TIP FAKÜLTESİ"
}
],
"authenticated-orcid": true,
"family": "ÇAPRAZ",
"given": "Mustafa",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4525-3477",
"affiliation": [
{
"name": "Sağlık Bilimleri Üniversitesi, Ankara Dışkapı Yıldırım Beyazıt Eğitim ve Araştırma Hastanesi,"
}
],
"authenticated-orcid": true,
"family": "ÖZTÜRK",
"given": "Alpaslan",
"sequence": "additional"
}
],
"container-title": "Sabuncuoglu Serefeddin Health Sciences",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
12,
19
]
],
"date-time": "2022-12-19T08:38:41Z",
"timestamp": 1671439121000
},
"deposited": {
"date-parts": [
[
2023,
6,
22
]
],
"date-time": "2023-06-22T04:11:08Z",
"timestamp": 1687407068000
},
"indexed": {
"date-parts": [
[
2023,
6,
23
]
],
"date-time": "2023-06-23T07:41:01Z",
"timestamp": 1687506061140
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2023,
1,
3
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2023,
1,
3
]
]
}
},
"member": "7009",
"original-title": [],
"page": "14-20",
"prefix": "10.55895",
"published": {
"date-parts": [
[
2023,
1,
3
]
]
},
"published-online": {
"date-parts": [
[
2023,
1,
3
]
]
},
"publisher": "Amasya University",
"reference": [
{
"DOI": "10.1515/dx-2020-0057",
"doi-asserted-by": "crossref",
"key": "ref1",
"unstructured": "Ciaccio, M., & Agnello, L. (2020). Biochemical biomarkers alterations in Coronavirus Disease 2019 \r\n(COVID-19). Diagnosis (Berl), 7(4), 365-372. doi:10.1515/dx-2020-0057"
},
{
"DOI": "10.1182/blood.2020006000",
"doi-asserted-by": "crossref",
"key": "ref2",
"unstructured": "Connors, J. M., & Levy, J. H. (2020). COVID-19 and its implications for thrombosis and \r\nanticoagulation. Blood, 135(23), 2033-2040. doi:10.1182/blood.2020006000"
},
{
"DOI": "10.1038/s41577-022-00762-9",
"doi-asserted-by": "crossref",
"key": "ref3",
"unstructured": "Conway, E. M., Mackman, N., Warren, R. Q., Wolberg, A. S., Mosnier, L. O., Campbell, R. A., . . . \r\nMorrissey, J. H. (2022). Understanding COVID-19-associated coagulopathy. Nat Rev Immunol, \r\n22(10), 639-649. doi:10.1038/s41577-022-00762-9"
},
{
"DOI": "10.1016/j.genrep.2021.101448",
"doi-asserted-by": "crossref",
"key": "ref4",
"unstructured": "Mir, S. M., Tahamtan, A., Nikoo, H. R., Arabi, M. S., Moradi, A. W., Ardakanian, S., & Tabarraei, A. \r\n(2022). Evaluation of biochemical characteristics of 183 COVID-19 patients: A retrospective \r\nstudy. Gene Rep, 26, 101448. doi:10.1016/j.genrep.2021.101448"
},
{
"DOI": "10.1111/jth.14879",
"doi-asserted-by": "crossref",
"key": "ref5",
"unstructured": "Thachil, J. (2020). What do monitoring platelet counts in COVID-19 teach us? Journal of \r\nThrombosis and Haemostasis, 18(8), 2071-2072. doi:10.1111/jth.14879"
},
{
"DOI": "10.52142/omujecm.39.1.31",
"doi-asserted-by": "crossref",
"key": "ref6",
"unstructured": "Tuğçe Atcali , S. Y., Cüneyt Çağlayan , Aykut Ulucan , Adem Kara. (2021). Effects of favipiravir on \r\nhematologic parameters and bone marrow in the rats. J Exp Clin Med, 39(1), 156-159."
},
{
"DOI": "10.1590/1806-9282.66.s2.65",
"doi-asserted-by": "crossref",
"key": "ref7",
"unstructured": "Yaylaci, S., Dheir, H., Senocak, D., Genc, A. B., Kocayigit, H., Cekic, D., . . . Karabay, O. (2020). The \r\neffects of favipiravir on hematological parameters of covid-19 patients. Rev Assoc Med Bras \r\n(1992), 66Suppl 2(Suppl 2), 65-70. doi:10.1590/1806-9282.66.S2.65\r\nZhou, F., Yu, T., Du, R., Fan, G., Liu, Y., Liu, Z., . . . Cao, B. (2020). Clinical course and risk factors for \r\nmortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. \r\nLancet, 395(10229), 1054-1062. doi:10.1016/S0140-6736(20)30566-3"
}
],
"reference-count": 7,
"references-count": 7,
"relation": {},
"resource": {
"primary": {
"URL": "http://dergipark.org.tr/en/doi/10.55895/sshs.1213382"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Effect of favipiravir use on INR, PT, aPTT tests of COVID-19 patients",
"type": "journal-article",
"volume": "4"
}