Impact of Molnupiravir Treatment on Patient-Reported Coronavirus Disease 2019 (COVID-19) Symptoms in the Phase 3 MOVe-OUT Trial: A Randomized, Placebo-Controlled Trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciad409, MOVe-OUT, NCT04575597, Jul 2023
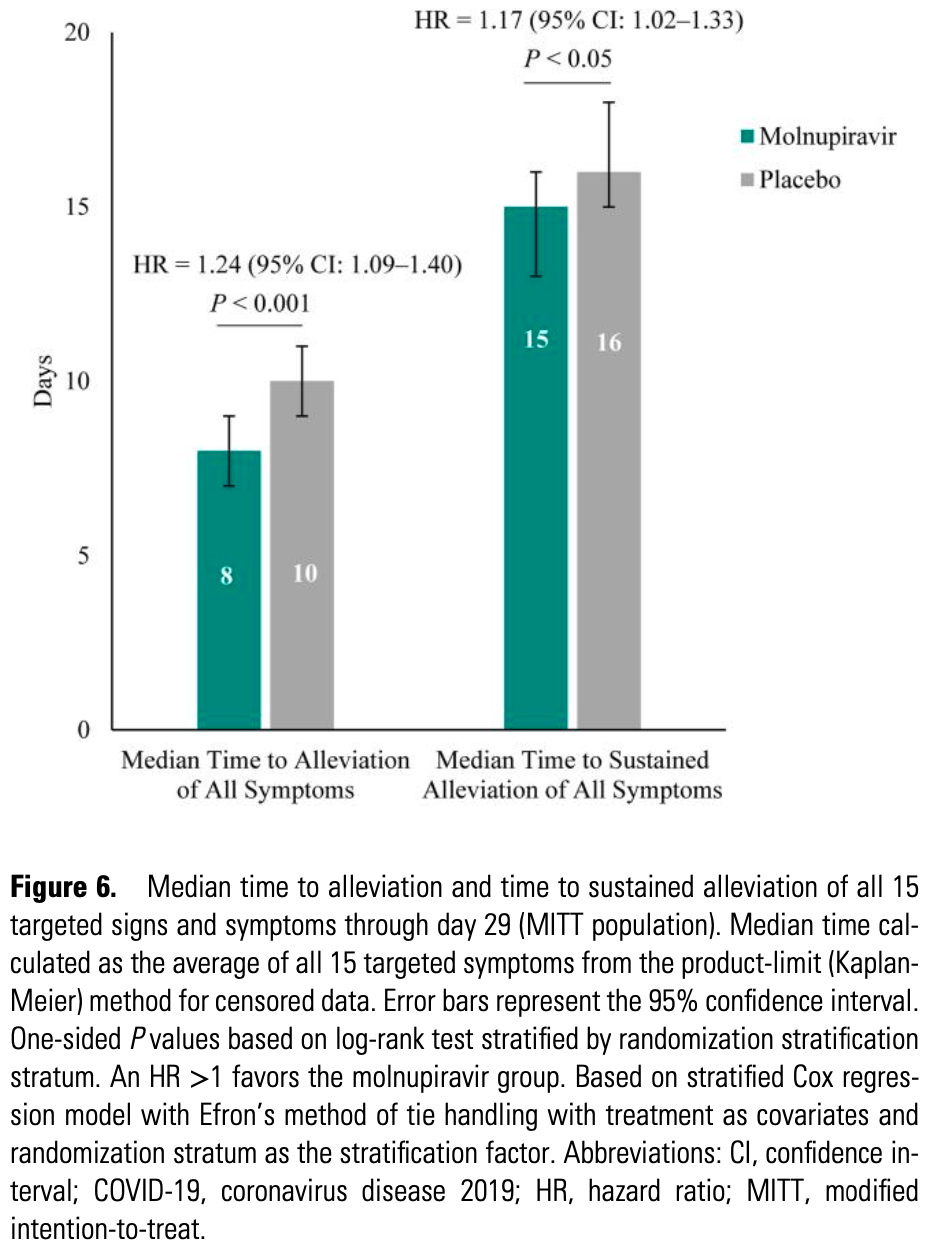
Symptom recovery details for the MOVe-OUT trial. Results are shown with the main paper1.
Potential risks of molnupiravir include the creation of dangerous variants, and mutagenicity, carcinogenicity, teratogenicity, and embryotoxicity2-16. Multiple analyses have identified variants potentially created by molnupiravir17-21. Studies show significantly increased risk of acute kidney injury22, cardiovascular toxocity23, and neurological symptoms22. Treatment may increase viral rebound24,25.
1.
Jayk Bernal et al., Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients, New England Journal of Medicine, doi:10.1056/NEJMoa2116044.
2.
Swanstrom et al., Lethal mutagenesis as an antiviral strategy, Science, doi:10.1126/science.abn0048.
3.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
4.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
5.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
6.
Huntsman, M., An assessment of the reproductive toxicity of the anti-COVID-19 drug molnupiravir using stem cell-based embryo models, Master's Thesis, scholarspace.manoa.hawaii.edu/items/cd11342c-b4dc-44c0-8b44-ce6e3369c40b.
7.
Huntsman (B) et al., Detection of developmental toxicity of the anti-COVID-19 drug molnupiravir using gastruloid-based in vitro assays, Toxicological Sciences, doi:10.1093/toxsci/kfaf093.
8.
Zibat et al., N4-hydroxycytidine, the active compound of Molnupiravir, promotes SARS-CoV-2 mutagenesis and escape from a neutralizing nanobody, iScience, doi:10.1016/j.isci.2023.107786.
9.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
10.
Gruber et al., Molnupiravir increases SARS‐CoV‐2 genome diversity and complexity: A case‐control cohort study, Journal of Medical Virology, doi:10.1002/jmv.29642.
11.
Marikawa et al., An active metabolite of the anti-COVID-19 drug molnupiravir impairs mouse preimplantation embryos at clinically relevant concentrations, Reproductive Toxicology, doi:10.1016/j.reprotox.2023.108475.
12.
Rahman, M., Elucidation of the DNA repair mechanisms involved in the repair of DNA damage caused by the Arabinosides and Anti-COVID-19 drugs, tokyo-metro-u.repo.nii.ac.jp/records/2000972.
13.
Zhou et al., β-D-N4-hydroxycytidine Inhibits SARS-CoV-2 Through Lethal Mutagenesis But Is Also Mutagenic To Mammalian Cells, The Journal of Infectious Diseases, doi:10.1093/infdis/jiab247.
14.
Chamod et al., Molnupiravir Metabolite--N4-hydroxycytidine Causes Cytotoxicity and DNA Damage in Mammalian Cells in vitro: N4-hydroxycytidine Induced Cytotoxicity DNA Damage, Asian Medical Journal and Alternative Medicine, 23:3, asianmedjam.com/index.php/amjam/article/view/1448.
15.
Standing et al., Randomized controlled trial of molnupiravir SARS-CoV-2 viral and antibody response in at-risk adult outpatients, Nature Communications, doi:10.1038/s41467-024-45641-0.
16.
Mori et al., Reactive oxygen species-mediated cytotoxic and DNA-damaging mechanism of N4-hydroxycytidine, a metabolite of the COVID-19 therapeutic drug molnupiravir, Free Radical Research, doi:10.1080/10715762.2025.2469738.
17.
Focosi et al., The fitness of molnupiravir-signed SARS-CoV-2 variants: imputation analysis based on prescription counts and GISAID analyses by country, Intervirology, doi:10.1159/000540282.
18.
Sanderson et al., A molnupiravir-associated mutational signature in global SARS-CoV-2 genomes, Nature, doi:10.1038/s41586-023-06649-6.
19.
Fountain-Jones et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, The Lancet Microbe, doi:10.1016/S2666-5247(23)00393-2.
20.
Kosakovsky Pond et al., Anti-COVID drug accelerates viral evolution, Nature, doi:10.1038/d41586-023-03248-3.
22.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
23.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
Guan et al., 19 Jul 2023, Double Blind Randomized Controlled Trial, placebo-controlled, multiple countries, peer-reviewed, 18 authors, study period May 2021 - November 2021, average treatment delay 4.0 days, trial NCT04575597 (history) (MOVe-OUT).
Contact: yanfen_guan@merck.com.
Impact of Molnupiravir Treatment on Patient-Reported Coronavirus Disease 2019 (COVID-19) Symptoms in the Phase 3 MOVe-OUT Trial: A Randomized, Placebo-Controlled Trial
Clinical Infectious Diseases, doi:10.1093/cid/ciad409
Background. Molnupiravir is an orally administered antiviral authorized for COVID-19 treatment in adults at high risk of progression to severe disease. Here, we report secondary and post hoc analyses of participants' self-reported symptoms in the MOVe-OUT trial, which evaluated molnupiravir initiated within 5 days of symptom onset in nonhospitalized, unvaccinated adults with mild-to-moderate, laboratory-confirmed COVID-19. Methods. Eligible participants completed a 15-item symptom diary daily from day 1 (randomization) through day 29, rating symptom severity as "none," "mild," "moderate," or "severe"; loss of smell and loss of taste were rated as "yes" or "no." Time to sustained symptom resolution/improvement was defined as the number of days from randomization to the first of 3 consecutive days of reduced severity, without subsequent relapse. Time to symptom progression was defined as the number of days from randomization to the first of 2 consecutive days of worsening severity. The Kaplan-Meier method was used to estimate event rates at various time points. The Cox proportional hazards model was used to estimate the hazard ratio between molnupiravir and placebo. Results. For most targeted COVID-19 symptoms, sustained resolution/improvement was more likely, and progression was less likely, in the molnupiravir versus placebo group through day 29. When evaluating 5 distinctive symptoms of COVID-19, molnupiravir participants had a shorter median time to first resolution (18 vs 20 d) and first alleviation (13 vs 15 d) of symptoms compared with placebo. Conclusions. Molnupiravir treatment in at-risk, unvaccinated patients resulted in improved clinical outcomes for most participant-reported COVID-19 symptoms compared with placebo. Clinical Trials Registration. ClinicalTrials.gov: NCT04575597.
References
Abdelnabi, Foo, Jonghe, Maes, Weynand et al., Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model, J Infect Dis
Adjei, Hong, Molinari, Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and Delta variant pandemic periods -United States, MMWR Morb Mortal Wkly Rep
Amdal, Taylor, Kuliś, Health-related quality of life in patients with COVID-19; international development of a patient-reported outcome measure, J Patient Rep Outcomes
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Butler, Hobbs, Gbinigie, Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial, Lancet
Caraco, Crofoot, Moncada, Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults, NEJM Evidence
Danza, Koo, Haddix, SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B. 1.1. 529 (omicron) variant predominance, MMWR Morb Mortal Wkly Rep
Jassat, Karim, Mudara, Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study, Lancet Glob Health
Johnson, Puenpatom, Moncada, Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial, Ann Intern Med
Kluzek, Dean, Wartolowska, Patient-reported outcome measures (PROMs) as proof of treatment efficacy, BMJ Evid Based Med
Madhi, Kwatra, Myers, Population immunity and COVID-19 severity with omicron variant in South Africa, N Engl J Med
Mercieca-Bebber, King, Calvert, Stockler, Friedlander, The importance of patient-reported outcomes in clinical trials and strategies for future optimization, Patient Relat Outcome Meas
Pourkarim, Pourtaghi-Anvarian, Rezaee, Molnupiravir: a new candidate for COVID-19 treatment, Pharmacol Res Perspect
Romano, Fehnel, Stoddard, Development of a novel patient-reported outcome measure to assess signs and symptoms of COVID-19, J Patient Rep Outcomes
Sheahan, Sims, Zhou, An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice, Sci Transl Med
Takashita, Kinoshita, Yamayoshi, Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2, N Engl J Med
Takashita, Yamayoshi, Simon, Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants, N Engl J Med
Wahl, Gralinski, Johnson, SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801, Nature
Watson, Barnsley, Toor, Hogan, Winskill et al., Global impact of the first year of COVID-19 vaccination: a mathematical modelling study, Lancet Infect Dis
Wong, Shah, Johnston, Carlsten, Ryerson, Patient-reported outcome measures after COVID-19: a prospective cohort study, Eur Respir J
Yoon, Toots, Lee, Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses, Antimicrob Agents Chemother
DOI record:
{
"DOI": "10.1093/cid/ciad409",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciad409",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Molnupiravir is an orally administered antiviral authorized for COVID-19 treatment in adults at high risk of progression to severe disease. Here, we report secondary and post hoc analyses of participants’ self-reported symptoms in the MOVe-OUT trial, which evaluated molnupiravir initiated within 5 days of symptom onset in nonhospitalized, unvaccinated adults with mild-to-moderate, laboratory-confirmed COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Eligible participants completed a 15-item symptom diary daily from day 1 (randomization) through day 29, rating symptom severity as “none,” “mild,” “moderate,” or “severe”; loss of smell and loss of taste were rated as “yes” or “no.” Time to sustained symptom resolution/improvement was defined as the number of days from randomization to the first of 3 consecutive days of reduced severity, without subsequent relapse. Time to symptom progression was defined as the number of days from randomization to the first of 2 consecutive days of worsening severity. The Kaplan-Meier method was used to estimate event rates at various time points. The Cox proportional hazards model was used to estimate the hazard ratio between molnupiravir and placebo.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>For most targeted COVID-19 symptoms, sustained resolution/improvement was more likely, and progression was less likely, in the molnupiravir versus placebo group through day 29. When evaluating 5 distinctive symptoms of COVID-19, molnupiravir participants had a shorter median time to first resolution (18 vs 20 d) and first alleviation (13 vs 15 d) of symptoms compared with placebo.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Molnupiravir treatment in at-risk, unvaccinated patients resulted in improved clinical outcomes for most participant-reported COVID-19 symptoms compared with placebo.</jats:p>\n <jats:p>Clinical Trials Registration. ClinicalTrials.gov: NCT04575597.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Guan",
"given": "Yanfen",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Puenpatom",
"given": "Amy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Johnson",
"given": "Matthew G",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Zhang",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Zhao",
"given": "Yujie",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centricity Research , Columbus, Georgia , USA"
}
],
"family": "Surber",
"given": "Joseph",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Carbon Health Technologies, Inc , North Hollywood, California , USA"
}
],
"family": "Weinberg",
"given": "Aaron",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "EAP Sardenya, Biomedical Research Institute Sant Pau , Barcelona , Spain"
}
],
"family": "Brotons",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Smolensk State Medical University , Smolensk , Russia"
}
],
"family": "Kozlov",
"given": "Roman",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clínica Médica Especialista en Pediatría e Infectología Pediátrica , Guatemala City , Guatemala"
}
],
"family": "Lopez",
"given": "Rudy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Paarl Research Centre , Paarl , South Africa"
}
],
"family": "Coetzee",
"given": "Kathleen",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Philippine General Hospital, University of the Philippines Manila , Manila , Philippines"
}
],
"family": "Santiaguel",
"given": "Joel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Du",
"given": "Jiejun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Williams-Diaz",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Brown",
"given": "Michelle",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Paschke",
"given": "Amanda",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "De Anda",
"given": "Carisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Merck & Co, Inc , Rahway, New Jersey , USA"
}
],
"family": "Norquist",
"given": "Josephine M",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
7,
19
]
],
"date-time": "2023-07-19T13:33:58Z",
"timestamp": 1689773638000
},
"deposited": {
"date-parts": [
[
2023,
7,
19
]
],
"date-time": "2023-07-19T23:58:24Z",
"timestamp": 1689811104000
},
"funder": [
{
"name": "Merck, Sharp & Dohme LLC"
}
],
"indexed": {
"date-parts": [
[
2023,
7,
20
]
],
"date-time": "2023-07-20T04:39:48Z",
"timestamp": 1689827988436
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
7,
19
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
7,
19
]
],
"date-time": "2023-07-19T00:00:00Z",
"timestamp": 1689724800000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciad409/50913511/ciad409.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciad409/50913511/ciad409.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"prefix": "10.1093",
"published": {
"date-parts": [
[
2023,
7,
19
]
]
},
"published-online": {
"date-parts": [
[
2023,
7,
19
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1093/infdis/jiab361",
"article-title": "Molnupiravir inhibits replication of the emerging SARS-CoV-2 variants of concern in a hamster infection model",
"author": "Abdelnabi",
"doi-asserted-by": "crossref",
"first-page": "749",
"journal-title": "J Infect Dis",
"key": "2023071919550719200_ciad409-B1",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1002/prp2.909",
"article-title": "Molnupiravir: a new candidate for COVID-19 treatment",
"author": "Pourkarim",
"doi-asserted-by": "crossref",
"first-page": "e00909",
"journal-title": "Pharmacol Res Perspect",
"key": "2023071919550719200_ciad409-B2",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"article-title": "An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice",
"author": "Sheahan",
"doi-asserted-by": "crossref",
"journal-title": "Sci Transl Med",
"key": "2023071919550719200_ciad409-B3",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2201933",
"article-title": "Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "1475",
"journal-title": "N Engl J Med",
"key": "2023071919550719200_ciad409-B4",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMc2207519",
"article-title": "Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants",
"author": "Takashita",
"doi-asserted-by": "crossref",
"first-page": "468",
"journal-title": "N Engl J Med",
"key": "2023071919550719200_ciad409-B5",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03312-w",
"article-title": "SARS-CoV-2 infection is effectively treated and prevented by EIDD-2801",
"author": "Wahl",
"doi-asserted-by": "crossref",
"first-page": "451",
"journal-title": "Nature",
"key": "2023071919550719200_ciad409-B6",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1128/AAC.00766-18",
"article-title": "Orally efficacious broad-spectrum ribonucleoside analog inhibitor of influenza and respiratory syncytial viruses",
"author": "Yoon",
"doi-asserted-by": "crossref",
"journal-title": "Antimicrob Agents Chemother",
"key": "2023071919550719200_ciad409-B7",
"volume": "62",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2023071919550719200_ciad409-B8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.2147/PROM.S156279",
"article-title": "The importance of patient-reported outcomes in clinical trials and strategies for future optimization",
"author": "Mercieca-Bebber",
"doi-asserted-by": "crossref",
"first-page": "353",
"journal-title": "Patient Relat Outcome Meas",
"key": "2023071919550719200_ciad409-B9",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1136/bmjebm-2020-111573",
"article-title": "Patient-reported outcome measures (PROMs) as proof of treatment efficacy",
"author": "Kluzek",
"doi-asserted-by": "crossref",
"first-page": "153",
"journal-title": "BMJ Evid Based Med",
"key": "2023071919550719200_ciad409-B10",
"volume": "27",
"year": "2022"
},
{
"DOI": "10.1183/13993003.03276-2020",
"article-title": "Patient-reported outcome measures after COVID-19: a prospective cohort study",
"author": "Wong",
"doi-asserted-by": "crossref",
"journal-title": "Eur Respir J",
"key": "2023071919550719200_ciad409-B11",
"volume": "56",
"year": "2020"
},
{
"DOI": "10.15585/mmwr.mm7137a4",
"article-title": "Mortality risk among patients hospitalized primarily for COVID-19 during the omicron and Delta variant pandemic periods—United States, April 2020-June 2022",
"author": "Adjei",
"doi-asserted-by": "crossref",
"first-page": "1182",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2023071919550719200_ciad409-B12",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1016/S2214-109X(22)00114-0",
"article-title": "Clinical severity of COVID-19 in patients admitted to hospital during the omicron wave in South Africa: a retrospective observational study",
"author": "Jassat",
"doi-asserted-by": "crossref",
"first-page": "e961",
"journal-title": "Lancet Glob Health",
"key": "2023071919550719200_ciad409-B13",
"volume": "10",
"year": "2022"
},
{
"author": "US Food and Drug Administration.",
"key": "2023071919550719200_ciad409-B14",
"year": "2020"
},
{
"DOI": "10.1056/EVIDoa2100043",
"article-title": "Phase 2/3 trial of molnupiravir for treatment of COVID-19 in nonhospitalized adults",
"author": "Caraco",
"doi-asserted-by": "crossref",
"journal-title": "NEJM Evidence",
"key": "2023071919550719200_ciad409-B15",
"volume": "1",
"year": "2022"
},
{
"author": "Centers for Disease Control and Prevention",
"key": "2023071919550719200_ciad409-B16",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)02597-1",
"article-title": "Molnupiravir plus usual care versus usual care alone as early treatment for adults with COVID-19 at increased risk of adverse outcomes (PANORAMIC): an open-label, platform-adaptive randomised controlled trial",
"author": "Butler",
"doi-asserted-by": "crossref",
"first-page": "281",
"journal-title": "Lancet",
"key": "2023071919550719200_ciad409-B17",
"volume": "401",
"year": "2023"
},
{
"DOI": "10.7326/M22-0729",
"article-title": "Effect of molnupiravir on biomarkers, respiratory interventions, and medical services in COVID-19: a randomized, placebo-controlled trial",
"author": "Johnson",
"doi-asserted-by": "crossref",
"first-page": "1126",
"journal-title": "Ann Intern Med",
"key": "2023071919550719200_ciad409-B18",
"volume": "175",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00320-6",
"article-title": "Global impact of the first year of COVID-19 vaccination: a mathematical modelling study",
"author": "Watson",
"doi-asserted-by": "crossref",
"first-page": "1293",
"journal-title": "Lancet Infect Dis",
"key": "2023071919550719200_ciad409-B19",
"volume": "22",
"year": "2022"
},
{
"article-title": "Population immunity and COVID-19 severity with omicron variant in South Africa",
"author": "Madhi",
"first-page": "1314",
"key": "2023071919550719200_ciad409-B20",
"volume-title": "N Engl J Med",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7105e1",
"article-title": "SARS-CoV-2 infection and hospitalization among adults aged ≥18 years, by vaccination status, before and during SARS-CoV-2 B. 1.1. 529 (omicron) variant predominance—Los Angeles County, California, November 7, 2021–January 8, 2022",
"author": "Danza",
"doi-asserted-by": "crossref",
"first-page": "177",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "2023071919550719200_ciad409-B21",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1186/s41687-022-00434-1",
"article-title": "Health-related quality of life in patients with COVID-19; international development of a patient-reported outcome measure",
"author": "Amdal",
"doi-asserted-by": "crossref",
"first-page": "26",
"journal-title": "J Patient Rep Outcomes",
"key": "2023071919550719200_ciad409-B22",
"volume": "6",
"year": "2022"
},
{
"DOI": "10.1186/s41687-022-00471-w",
"article-title": "Development of a novel patient-reported outcome measure to assess signs and symptoms of COVID-19",
"author": "Romano",
"doi-asserted-by": "crossref",
"first-page": "85",
"journal-title": "J Patient Rep Outcomes",
"key": "2023071919550719200_ciad409-B23",
"volume": "6",
"year": "2022"
}
],
"reference-count": 23,
"references-count": 23,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/advance-article/doi/10.1093/cid/ciad409/7226391"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Impact of Molnupiravir Treatment on Patient-Reported Coronavirus Disease 2019 (COVID-19) Symptoms in the Phase 3 MOVe-OUT Trial: A Randomized, Placebo-Controlled Trial",
"type": "journal-article"
}