The efficacy of corticosteroids therapy in patients with moderate to severe SARS-CoV-2 infection: a multicenter, randomized, open-label trial
et al., Respiratory Research, doi:10.1186/s12931-021-01833-6, Sep 2021
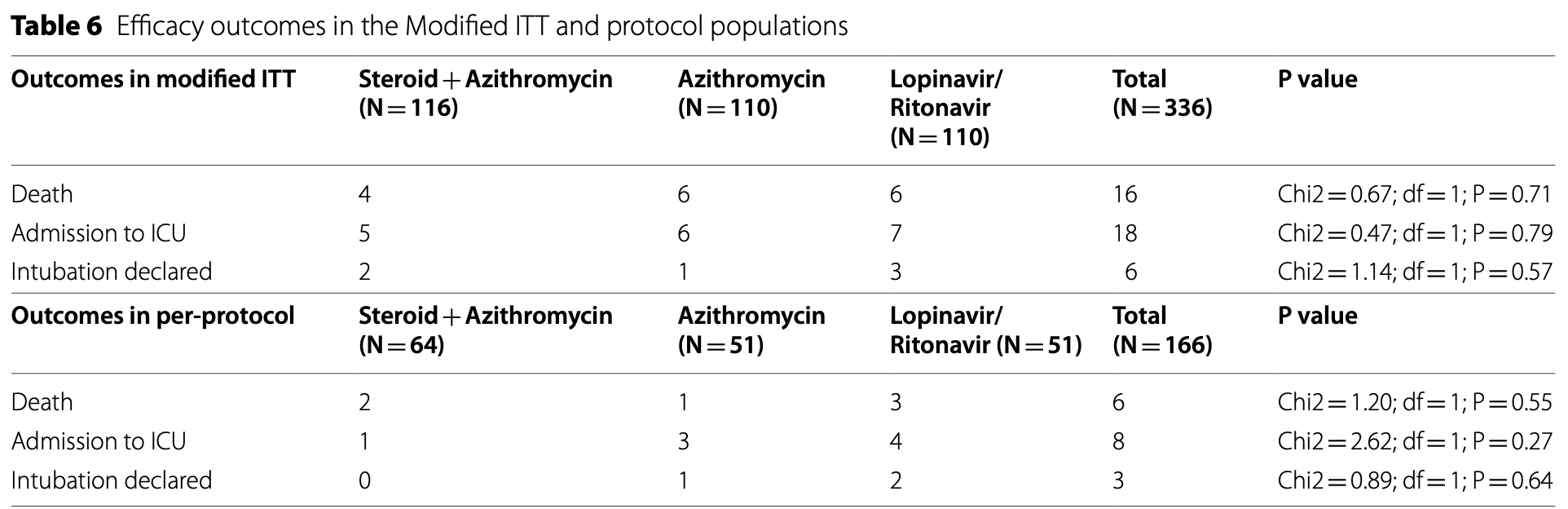
RCT 336 COVID-19 patients with moderate to severe infection showing low-dose prednisolone significantly reduced length of hospital stay compared to lopinavir/ritonavir. There were no significant differences when comparing azithromycin to lopinavir/ritonavir.
|
risk of death, 18.9% lower, RR 0.81, p = 0.79, treatment 10 of 226 (4.4%), control 6 of 110 (5.5%), NNT 97, azithromycin groups vs. lopinavir/ritonavir.
|
|
risk of mechanical ventilation, 51.3% lower, RR 0.49, p = 0.40, treatment 3 of 226 (1.3%), control 3 of 110 (2.7%), NNT 71, azithromycin groups vs. lopinavir/ritonavir.
|
|
risk of ICU admission, 23.5% lower, RR 0.76, p = 0.61, treatment 11 of 226 (4.9%), control 7 of 110 (6.4%), NNT 67, azithromycin groups vs. lopinavir/ritonavir.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Ghanei et al., 15 Sep 2021, Randomized Controlled Trial, Iran, peer-reviewed, 18 authors, study period 13 April, 2020 - 9 August, 2020.
Contact: mghaneister@gmail.com (corresponding author).
The efficacy of corticosteroids therapy in patients with moderate to severe SARS-CoV-2 infection: a multicenter, randomized, open-label trial
Respiratory Research, doi:10.1186/s12931-021-01833-6
Background: We performed a multicenter, randomized open-label trial in patients with moderate to severe Covid-19 treated with a range of possible treatment regimens. Methods: Patients were randomly assigned to one of three regimen groups at a ratio of 1:1:1. The primary outcome of this study was admission to the intensive care unit. Secondary outcomes were intubation, in-hospital mortality, time to clinical recovery, and length of hospital stay (LOS). Between April 13 and August 9, 2020, a total of 336 patients were randomly assigned to receive one of the 3 treatment regimens including group I (hydroxychloroquine stat, prednisolone, azithromycin and naproxen; 120 patients), group II (hydroxychloroquine stat, azithromycin and naproxen; 116 patients), and group III (hydroxychloroquine and lopinavir/ ritonavir (116 patients). The mean LOS in patients receiving prednisolone was 5.5 in the modified intention-to-treat (mITT) population and 4.4 days in the per-protocol (PP) population compared with 6.4 days (mITT population) and 5.8 days (PP population) in patients treated with Lopinavir/Ritonavir.
Results: The mean LOS was significantly lower in the mITT and PP populations who received prednisolone compared with populations treated with Lopinavir/Ritonavir (p = 0.028; p = 0.0007). We observed no significant differences in the number of deaths, ICU admission, and need for mechanical ventilation between the Modified ITT and per-protocol populations treated with prednisolone and Lopinavir/Ritonavir, although these outcomes were better in the arm treated with prednisolone. The time to clinical recovery was similar in the modified ITT and per-protocol populations treated with prednisolone, lopinavir/ritonavir, and azithromycin (P = 0.335; P = 0.055; p = 0.291; p = 0.098).
Conclusion: The results of the present study show that therapeutic regimen (regimen I) with low dose prednisolone was superior to other regimens in shortening the length of hospital stay in patients with moderate to severe COVID-19. The steroid sparing effect may be utilized to increase the effectiveness of corticosteroids in the management of diabetic patients by decreasing the dosage.
Abbreviations LOS: Length of hospital stay; mITT: Modified intention-to-treat; ICU: Intensive care unit; NSAIDs: Nonsteroidal anti-inflammatory drugs; DSMB: Data and Safety Monitoring Board; AFOP: Acute fibrinous and organizing pneumonia; PH: Proportional hazard,; GEE: General Estimation Equation. Authors' contributions MG, MSD, AQ, AHG, SAS, SHS, HG, SH, ME, AHS, YI, EV, FB, FTR, MHAA, AA, and FFG in designed the study, performed the experiments, analyzed the results, wrote the article, revised it. All authors read and approved the final manuscript.
Declarations Ethics approval and consent to participate The study protocol was approved by the Baqiyatallah and Iran Universities of Medical Sciences. This trial was also approved by the Independent Ethics Committees of both universities (The trial number, IRCT20200318046812N2; https:// fa. irct. ir/ trial/ 46968 ). Written informed consent was obtained from all the participants.
Consent to publication Authors are agree to publication.
Competing interests The authors declare that they have no conflict of interest.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Alessi, Oliveira, Schaan, Telo, Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes, Diabetol Metab Syndr
Apicella, Campopiano, Mantuano, Mazoni, Coppelli et al., COVID-19 in people with diabetes: understanding the reasons for worse outcomes, Lancet Diabetes Endocrinol
Bartoletti, Marconi, Scudeller, Pancaldi, Tedeschi et al., Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study, Clin Microbiol Infect
Buja, Wolf, Zhao, Akkanti, Mcdonald et al., The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities, Cardiovasc Pathol
Copin, Parmentier, Duburcq, Poissy, Mathieu, Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection, Intensive Care Med
De Simone, Mancusi, Finding the right time for anti-inflammatory therapy in COVID-19, Int J Infect Dis
Fadel, Morrison, Vahia, Smith, Chaudhry et al., Early short-course corticosteroids in hospitalized patients with COVID-19, Clin Infect Dis
Flikweert, Grootenboers, Yick, Du Mée, Van Der Meer et al., Late histopathologic characteristics of critically ill COVID-19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series, J Crit Care
Grasselli, Pesenti, Cecconi, Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response, JAMA
Guan, Ni, Hu, Liang, Ou et al., Clinical characteristics of coronavirus disease 2019 in China, N Engl J Med
Horby, Lim, Emberson, Mafham, Bell et al., Dexamethasone in hospitalized patients with Covid-19-preliminary report, N Engl J Med
Huang, Wang, Li, Ren, Zhao et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet
Jamilloux, Henry, Belot, Viel, Fauter et al., Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions, Autoimmun Rev
Jeronimo, Farias, Val, Sampaio, Alexandre et al., Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial, Clin Infect Dis, doi:10.1093/cid/ciaa1177
Jin, Cai, Cheng, Cheng, Deng et al., A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version), Mil Med Res
Mason, Pathogenesis of COVID-19 from a cell biology perspective, Eur Respir J
Mattos-Silva, Felix, Silva, Robba, Battaglini et al., Pros and cons of corticosteroid therapy for COVID-19 patients, Respir Physiol Neurobiol
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Mohme, Iran's National Guideline for 2019-nCoV
Russell, Millar, Baillie, Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury, Lancet
Shang, Zhao, Hu, Du, Cao, On the use of corticosteroids for 2019-nCoV pneumonia, Lancet
Wang, Jiang, He, Wang, Wang et al., A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia, Signal Transduct Target Ther
Wang, Jiang, He, Wang, Wang et al., Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan China, MedRxiv
Wu, Li, Shi, Chen, Jiang et al., Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19), J Intern Med
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir Med
Yang, Yu, Xu, Shu, Liu et al., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study, Lancet Respir Med
Ye, Wang, Mao, The pathogenesis and treatment of theCytokine Storm'in COVID-19, J Infect
DOI record:
{
"DOI": "10.1186/s12931-021-01833-6",
"ISSN": [
"1465-993X"
],
"URL": "http://dx.doi.org/10.1186/s12931-021-01833-6",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>We performed a multicenter, randomized open-label trial in patients with moderate to severe Covid-19 treated with a range of possible treatment regimens. Methods: Patients were randomly assigned to one of three regimen groups at a ratio of 1:1:1. The primary outcome of this study was admission to the intensive care unit. Secondary outcomes were intubation, in-hospital mortality, time to clinical recovery, and length of hospital stay (LOS). Between April 13 and August 9, 2020, a total of 336 patients were randomly assigned to receive one of the 3 treatment regimens including group I (hydroxychloroquine stat, prednisolone, azithromycin and naproxen; 120 patients), group II (hydroxychloroquine stat, azithromycin and naproxen; 116 patients), and group III (hydroxychloroquine and lopinavir/ritonavir (116 patients). The mean LOS in patients receiving prednisolone was 5.5 in the modified intention-to-treat (mITT) population and 4.4 days in the per-protocol (PP) population compared with 6.4 days (mITT population) and 5.8 days (PP population) in patients treated with Lopinavir/Ritonavir.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>The mean LOS was significantly lower in the mITT and PP populations who received prednisolone compared with populations treated with Lopinavir/Ritonavir (p = 0.028; p = 0.0007). We observed no significant differences in the number of deaths, ICU admission, and need for mechanical ventilation between the Modified ITT and per-protocol populations treated with prednisolone and Lopinavir/Ritonavir, although these outcomes were better in the arm treated with prednisolone. The time to clinical recovery was similar in the modified ITT and per-protocol populations treated with prednisolone, lopinavir/ritonavir, and azithromycin (P = 0.335; P = 0.055; p = 0.291; p = 0.098).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>The results of the present study show that therapeutic regimen (regimen I) with low dose prednisolone was superior to other regimens in shortening the length of hospital stay in patients with moderate to severe COVID-19. The steroid sparing effect may be utilized to increase the effectiveness of corticosteroids in the management of diabetic patients by decreasing the dosage.</jats:p>\n </jats:sec>",
"alternative-id": [
"1833"
],
"article-number": "245",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "14 May 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "25 August 2021"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "15 September 2021"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study protocol was approved by the Baqiyatallah and Iran Universities of Medical Sciences. This trial was also approved by the Independent Ethics Committees of both universities (The trial number, IRCT20200318046812N2; ExternalRef removed). Written informed consent was obtained from all the participants."
},
{
"group": {
"label": "Consent to publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Authors are agree to publication."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no conflict of interest."
}
],
"author": [
{
"affiliation": [],
"family": "Ghanei",
"given": "Mostafa",
"sequence": "first"
},
{
"affiliation": [],
"family": "Solaymani-Dodaran",
"given": "Masoud",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qazvini",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghazale",
"given": "Amir Hosein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Setarehdan",
"given": "Seyed Amin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saadat",
"given": "Seyed Hassan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghobadi",
"given": "Hassan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoseininia",
"given": "Saeed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elahikhah",
"given": "Maryam",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Samadi",
"given": "Ali Hossein",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Imani",
"given": "Yousef",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vahedi",
"given": "Ensieh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Babamahmoodi",
"given": "Farhang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rostami",
"given": "Fatemeh Tajik",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ardebili",
"given": "Mohammad Hossein Azimzadeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ansarifar",
"given": "Akram",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Golmaei",
"given": "Fatemeh Fallahpoor",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Asadollah",
"given": "Atieh",
"sequence": "additional"
}
],
"container-title": "Respiratory Research",
"container-title-short": "Respir Res",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2021,
9,
15
]
],
"date-time": "2021-09-15T08:03:06Z",
"timestamp": 1631692986000
},
"deposited": {
"date-parts": [
[
2021,
9,
15
]
],
"date-time": "2021-09-15T08:07:35Z",
"timestamp": 1631693255000
},
"indexed": {
"date-parts": [
[
2025,
5,
4
]
],
"date-time": "2025-05-04T05:25:56Z",
"timestamp": 1746336356415
},
"is-referenced-by-count": 28,
"issue": "1",
"issued": {
"date-parts": [
[
2021,
9,
15
]
]
},
"journal-issue": {
"issue": "1",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
15
]
],
"date-time": "2021-09-15T00:00:00Z",
"timestamp": 1631664000000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
9,
15
]
],
"date-time": "2021-09-15T00:00:00Z",
"timestamp": 1631664000000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12931-021-01833-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12931-021-01833-6/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12931-021-01833-6.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2021,
9,
15
]
]
},
"published-online": {
"date-parts": [
[
2021,
9,
15
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1001/jama.2020.4031",
"author": "G Grasselli",
"doi-asserted-by": "publisher",
"first-page": "1545",
"journal-title": "JAMA",
"key": "1833_CR1",
"unstructured": "Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–6.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.carpath.2020.107233",
"author": "LM Buja",
"doi-asserted-by": "publisher",
"first-page": "107233",
"journal-title": "Cardiovasc Pathol.",
"key": "1833_CR2",
"unstructured": "Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233.",
"volume": "48",
"year": "2020"
},
{
"DOI": "10.1038/s41392-019-0089-y",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "1833_CR3",
"unstructured": "Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, et al. A retrospective cohort study of methylprednisolone therapy in severe patients with COVID-19 pneumonia. Signal Transduct Target Ther. 2020;5:1–3.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.autrev.2020.102567",
"author": "Y Jamilloux",
"doi-asserted-by": "publisher",
"first-page": "102567",
"journal-title": "Autoimmun Rev.",
"key": "1833_CR4",
"unstructured": "Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev. 2020;19:102567.",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"journal-title": "Lancet",
"key": "1833_CR5",
"unstructured": "Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2020.07.002",
"author": "AW Flikweert",
"doi-asserted-by": "publisher",
"first-page": "149",
"journal-title": "J Crit Care",
"key": "1833_CR6",
"unstructured": "Flikweert AW, Grootenboers MJJH, Yick DCY, du Mée AWF, van der Meer NJM, Rettig TCD, et al. Late histopathologic characteristics of critically ill COVID-19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149–55.",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"author": "Z Xu",
"doi-asserted-by": "publisher",
"first-page": "420",
"journal-title": "Lancet Respir Med",
"key": "1833_CR7",
"unstructured": "Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–2.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06057-8",
"author": "M-C Copin",
"doi-asserted-by": "publisher",
"first-page": "1124",
"journal-title": "Intensive Care Med",
"key": "1833_CR8",
"unstructured": "Copin M-C, Parmentier E, Duburcq T, Poissy J, Mathieu D. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–6.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"author": "X Yang",
"doi-asserted-by": "publisher",
"first-page": "475",
"journal-title": "Lancet Respir Med",
"key": "1833_CR9",
"unstructured": "Yang X, Yu Y, Xu J, Shu H, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"author": "W Guan",
"doi-asserted-by": "publisher",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "1833_CR10",
"unstructured": "Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet",
"key": "1833_CR11",
"unstructured": "Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30317-2",
"author": "CD Russell",
"doi-asserted-by": "publisher",
"first-page": "473",
"journal-title": "Lancet",
"key": "1833_CR12",
"unstructured": "Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–5.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30361-5",
"author": "L Shang",
"doi-asserted-by": "publisher",
"first-page": "683",
"journal-title": "Lancet (London, England)",
"key": "1833_CR13",
"unstructured": "Shang L, Zhao J, Hu Y, Du R, Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet (London, England). 2020;395:683.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1101/2020.06.22.20137273",
"doi-asserted-by": "crossref",
"key": "1833_CR14",
"unstructured": "Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E PB. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020."
},
{
"DOI": "10.1016/j.resp.2020.103492",
"author": "P Mattos-Silva",
"doi-asserted-by": "publisher",
"first-page": "103492",
"journal-title": "Respir Physiol Neurobiol.",
"key": "1833_CR15",
"unstructured": "Mattos-Silva P, Felix NS, Silva PL, Robba C, Battaglini D, Pelosi P, et al. Pros and cons of corticosteroid therapy for COVID-19 patients. Respir Physiol Neurobiol. 2020;280:103492.",
"volume": "280",
"year": "2020"
},
{
"author": "Y Wang",
"first-page": "1",
"journal-title": "MedRxiv.",
"key": "1833_CR16",
"unstructured": "Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, et al. Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan China. MedRxiv. 2020;1:1–17.",
"volume": "1",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa601",
"author": "R Fadel",
"doi-asserted-by": "publisher",
"first-page": "2114",
"journal-title": "Clin Infect Dis",
"key": "1833_CR17",
"unstructured": "Fadel R, Morrison AR, Vahia A, Smith ZR, Chaudhry Z, Bhargava P, et al. Early short-course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis. 2020;71:2114–20.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1186/s13098-019-0485-z",
"author": "J Alessi",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Diabetol Metab Syndr",
"key": "1833_CR18",
"unstructured": "Alessi J, De Oliveira GB, Schaan BD, Telo GH. Dexamethasone in the era of COVID-19: friend or foe? An essay on the effects of dexamethasone and the potential risks of its inadvertent use in patients with diabetes. Diabetol Metab Syndr. 2020;12:1–11.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/S2213-8587(20)30238-2",
"author": "M Apicella",
"doi-asserted-by": "publisher",
"first-page": "782",
"issue": "9",
"journal-title": "Lancet Diabetes Endocrinol.",
"key": "1833_CR19",
"unstructured": "Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8(9):782–92.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1177",
"author": "CMP Jeronimo",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "1833_CR20",
"unstructured": "Jeronimo CMP, Farias MEL, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciaa1177.",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2020.09.014",
"author": "M Bartoletti",
"doi-asserted-by": "publisher",
"first-page": "105",
"journal-title": "Clin Microbiol Infect",
"key": "1833_CR21",
"unstructured": "Bartoletti M, Marconi L, Scudeller L, Pancaldi L, Tedeschi S, Giannella M, et al. Efficacy of corticosteroid treatment for hospitalized patients with severe COVID-19: a multicentre study. Clin Microbiol Infect. 2021;27:105–11.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1183/13993003.00607-2020",
"author": "RJ Mason",
"doi-asserted-by": "publisher",
"first-page": "2000607",
"journal-title": "Eur Respir J",
"key": "1833_CR22",
"unstructured": "Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55:2000607.",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1111/joim.13063",
"author": "J Wu",
"doi-asserted-by": "publisher",
"first-page": "128",
"journal-title": "J Intern Med",
"key": "1833_CR23",
"unstructured": "Wu J, Li W, Shi X, Chen Z, Jiang B, Liu J, et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J Intern Med. 2020;288:128–38.",
"volume": "288",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.09.1454",
"author": "G de Simone",
"doi-asserted-by": "publisher",
"first-page": "247",
"journal-title": "Int J Infect Dis",
"key": "1833_CR24",
"unstructured": "de Simone G, Mancusi C. Finding the right time for anti-inflammatory therapy in COVID-19. Int J Infect Dis. 2020;101:247–8.",
"volume": "101",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.037",
"author": "Q Ye",
"doi-asserted-by": "publisher",
"first-page": "607",
"journal-title": "J Infect",
"key": "1833_CR25",
"unstructured": "Ye Q, Wang B, Mao J. The pathogenesis and treatment of theCytokine Storm’in COVID-19. J Infect. 2020;80:607–13.",
"volume": "80",
"year": "2020"
},
{
"author": "Y-H Jin",
"first-page": "1",
"journal-title": "Mil Med Res",
"key": "1833_CR26",
"unstructured": "Jin Y-H, Cai L, Cheng Z-S, Cheng H, Deng T, Fan Y-P, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7:1–23.",
"volume": "7",
"year": "2020"
},
{
"key": "1833_CR27",
"unstructured": "MOHME. Iran’s National Guideline for 2019-nCoV; 1st edition. In: MOHME. 2020 pp. 1–20. http://ird.behdasht.gov.ir/."
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://respiratory-research.biomedcentral.com/articles/10.1186/s12931-021-01833-6"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "The efficacy of corticosteroids therapy in patients with moderate to severe SARS-CoV-2 infection: a multicenter, randomized, open-label trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}
ghanei2