Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities
et al., Journal of Infection, doi:10.1016/j.jinf.2023.05.012, Aug 2023
Retrospective 2,118 hospitalized COVID-19 patients in China, showing improved results with azvudine vs. paxlovid.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
Study covers paxlovid and azvudine.
|
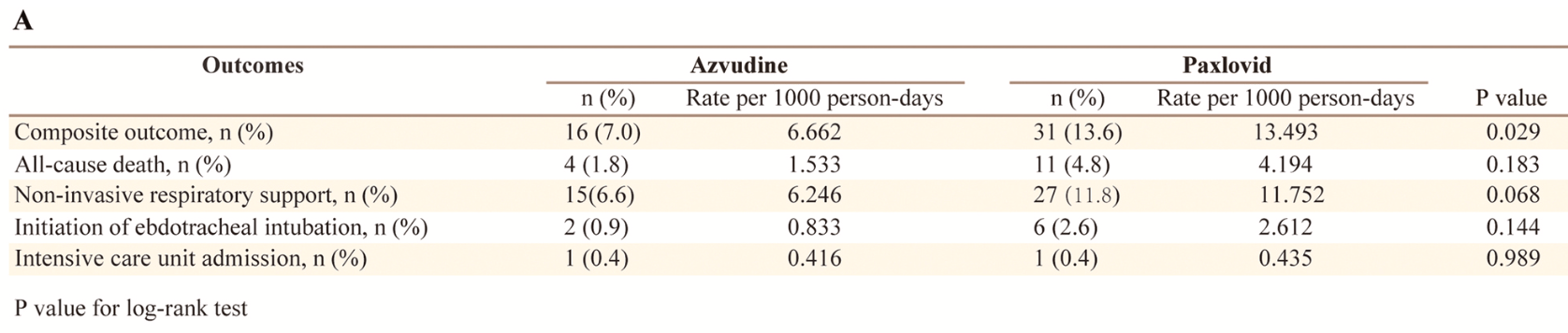
risk of death, 175.0% higher, RR 2.75, p = 0.11, treatment 11 of 228 (4.8%), control 4 of 228 (1.8%), propensity score matching.
|
|
risk of mechanical ventilation, 200.0% higher, RR 3.00, p = 0.28, treatment 6 of 228 (2.6%), control 2 of 228 (0.9%), propensity score matching.
|
|
risk of ICU admission, no change, RR 1.00, p = 1.00, treatment 1 of 228 (0.4%), control 1 of 228 (0.4%), propensity score matching.
|
|
composite outcome, 93.8% higher, RR 1.94, p = 0.03, treatment 31 of 228 (13.6%), control 16 of 228 (7.0%), non-invasive respiratory support, endotracheal intubation, ICU admission, all-cause death, propensity score matching.
|
|
respiratory suport, 80.0% higher, RR 1.80, p = 0.07, treatment 27 of 228 (11.8%), control 15 of 228 (6.6%), propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Dian et al., 31 Aug 2023, retrospective, China, peer-reviewed, 5 authors, study period 5 December, 2022 - 31 January, 2023, this trial compares with another treatment - results may be better when compared to placebo.
Abstract: Journal of Infection 87 (2023) e24–e27
Contents lists available at ScienceDirect
Journal of Infection
journal homepage: www.elsevier.com/locate/jinf
Letter to the Editor
Azvudine versus Paxlovid for oral treatment of
COVID-19 in Chinese patients with pre-existing
comorbidities
]]]]
]]
Dear Editor,
Chinese guidelines grant the use of Azvudine and Paxlovid in
COVID-19 patients, especially those with pre-existing comorbid
ities.1,2 Recently, Gao Y et al. reported that Paxlovid appears to be
superior to Azvudine in the virus clearance among general COVID-19
patients.3 However, a multicenter randomized controlled study de
monstrated that Paxlovid showed no significant reduction in the risk
of all-cause mortality on day 28 and the duration of virus clearance
in hospitalized adult COVID-19 patients with pre-existing co
morbidities.4 Several studies demonstrated that Azvudine could re
duce the duration of virus clearance and improve the clinical
prognosis in COVID-19 patients including those with pre-existing
comorbidities.5–8 Therefore, concerns arise about the clinical effec
tiveness of Azvudine versus Paxlovid in COVID-19 patients with preexisting comorbidities on admission.
Here, we conducted a single-center, retrospective cohort study
during the outbreak caused by the omicron from December 5,
2022 to January 31, 2023 in Xiangya Hospital of Central South
University. The study included hospitalized patients with preexisting comorbidities and confirmed diagnosis of SARS-CoV-2
infection who received Paxlovid or Azvudine. The patients with
these conditions were excluded: 1) younger than 18 years; 2) re
ceived oxygen support or mechanical ventilation on the date of
the admission; 3) not received any antiviral agents; 4) received
both Azvudine and Paxlovid. The study was approved by the in
stitutional review board of Xiangya Hospital of Central South
University, and all the patients were anonymous and no need for
individual informed consent.
The primary endpoint was a composite disease progression
outcome which was defined as any of the following events: 1) noninvasive respiratory support; 2) initiation of endotracheal intuba
tion; 3) intensive care unit admission; 4) all-cause death. The sec
ondary endpoints were each of these individual disease progression
outcomes. Patients were observed from the date of admission until
discharge, occurrence of outcome event or death, whichever came
first. We used propensity-score models conditional on baseline
characteristics, and the probability of receiving Azvudine was esti
mated in an approach of calliper matching without replacement,
with a calliper width of 0.2. The baseline covariates included age,
sex, time from symptom onset to hospitalization, COVID-19 vacci
nation status, severity of COVID-19 on admission (severe cases were
defined as having respiratory rate ≥30, or oxygen saturation ≤93%, or
PaO2/FiO2 ≤300 mmHg, or lung infiltrates > 50%), and concomitant
treatments initiated at admission (systemic steroid and antibiotics).
The standard mean differences (SMDs) were used to assess the
balance of each baseline covariates between the groups before and
after propensity-score matching which less than 0.1 indicating
covariate was balanced.9 The incidence rates of outcome events were
calculated as the number of outcome events / (sum of person ×
hospital days). Univariate Cox regression model was used to estimate
a hazard ratio (HR) with 95% confidence interval (CI) for each..
DOI record:
{
"DOI": "10.1016/j.jinf.2023.05.012",
"ISSN": [
"0163-4453"
],
"URL": "http://dx.doi.org/10.1016/j.jinf.2023.05.012",
"alternative-id": [
"S0163445323002906"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jinf.2023.05.012"
},
{
"label": "Content Type",
"name": "content_type",
"value": "simple-article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2023 The British Infection Association. Published by Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Dian",
"given": "Yating",
"sequence": "first"
},
{
"affiliation": [],
"family": "Meng",
"given": "Yu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Yuming",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Deng",
"given": "Guangtong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zeng",
"given": "Furong",
"sequence": "additional"
}
],
"container-title": "Journal of Infection",
"container-title-short": "Journal of Infection",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"journalofinfection.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2023,
5,
17
]
],
"date-time": "2023-05-17T09:07:19Z",
"timestamp": 1684314439000
},
"deposited": {
"date-parts": [
[
2023,
7,
21
]
],
"date-time": "2023-07-21T16:31:02Z",
"timestamp": 1689957062000
},
"funder": [
{
"DOI": "10.13039/501100001809",
"award": [
"82102803",
"82103183",
"82272849"
],
"doi-asserted-by": "publisher",
"name": "National Natural Science Foundation of China"
},
{
"DOI": "10.13039/501100004735",
"award": [
"2021JJ40976",
"2022JJ40767"
],
"doi-asserted-by": "publisher",
"name": "Natural Science Foundation of Hunan Province"
},
{
"DOI": "10.13039/501100011790",
"doi-asserted-by": "publisher",
"name": "Xiangya Hospital, Central South University"
}
],
"indexed": {
"date-parts": [
[
2023,
7,
22
]
],
"date-time": "2023-07-22T04:13:53Z",
"timestamp": 1689999233029
},
"is-referenced-by-count": 1,
"issue": "2",
"issued": {
"date-parts": [
[
2023,
8
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2023,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
8,
1
]
],
"date-time": "2023-08-01T00:00:00Z",
"timestamp": 1690848000000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323002906?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0163445323002906?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "e24-e27",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2023,
8
]
]
},
"published-print": {
"date-parts": [
[
2023,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"key": "10.1016/j.jinf.2023.05.012_bib1",
"unstructured": "General Office of the National Health Commission. Notice on the issuance of Diagnosis and Treatment Protocol for novel coronavirus infection (trial version 9); 2022. Accessed on: 〈http://www.gov.cn/zhengce/zhengceku/2022-03/15/content_5679257.htm〉."
},
{
"key": "10.1016/j.jinf.2023.05.012_bib2",
"unstructured": "General Office of the National Health Commission. Notice on the issuance of Diagnosis and Treatment Protocol for novel coronavirus infection (trial version 10); 2023. Accessed on: 〈http://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm〉."
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"article-title": "Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19",
"author": "Gao",
"doi-asserted-by": "crossref",
"first-page": "e158",
"issue": "6",
"journal-title": "J Infect",
"key": "10.1016/j.jinf.2023.05.012_bib3",
"volume": "86",
"year": "2023"
},
{
"article-title": "Efficacy and safety of Paxlovid in severe adult patients with SARS-Cov-2 infection: a multicenter randomized controlled study",
"author": "Liu",
"journal-title": "Lancet Reg Health West Pac",
"key": "10.1016/j.jinf.2023.05.012_bib4",
"volume": "33",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"article-title": "Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients",
"author": "Zhang",
"doi-asserted-by": "crossref",
"first-page": "414",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "10.1016/j.jinf.2023.05.012_bib5",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1002/advs.202001435",
"article-title": "A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study",
"author": "Ren",
"doi-asserted-by": "crossref",
"issue": "19",
"journal-title": "Adv Sci",
"key": "10.1016/j.jinf.2023.05.012_bib6",
"volume": "7",
"year": "2020"
},
{
"article-title": "Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 omicron variant: a retrospective cohort study",
"author": "Chen",
"journal-title": "medRxiv",
"key": "10.1016/j.jinf.2023.05.012_bib7",
"year": "2023"
},
{
"article-title": "Real-world effectiveness of Azvudine in hospitalized patients with COVID-19: a retrospective cohort study",
"author": "Shen",
"journal-title": "medRxiv",
"key": "10.1016/j.jinf.2023.05.012_bib8",
"year": "2023"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"article-title": "Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study",
"author": "Wong",
"doi-asserted-by": "crossref",
"first-page": "1681",
"issue": "12",
"journal-title": "Lancet Infect Dis",
"key": "10.1016/j.jinf.2023.05.012_bib9",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2204919",
"article-title": "Nirmatrelvir use and severe covid-19 outcomes during the omicron surge",
"author": "Arbel",
"doi-asserted-by": "crossref",
"first-page": "790",
"issue": "9",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jinf.2023.05.012_bib10",
"volume": "387",
"year": "2022"
}
],
"reference-count": 10,
"references-count": 10,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0163445323002906"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)"
],
"subtitle": [],
"title": "Azvudine versus Paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "87"
}
dian