Clinical Predictors for Abnormal ALT in Patients Infected with COVID-19—A Retrospective Single Centre Study
et al., Pathogens, doi:10.3390/pathogens12030473, Mar 2023
Retrospective 163 COVID-19 patients in Singapore, showing increased risk of liver injury (abnormal ALT) with acetaminophen in a dose-dependent manner, and with remdesivir, without statistical significance in both cases.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Study covers remdesivir and acetaminophen.
|
abnormal ALT, 68.0% higher, OR 1.68, p = 0.40, treatment 12, control 151, adjusted per study, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Chew et al., 16 Mar 2023, retrospective, Singapore, peer-reviewed, median age 56.0, 7 authors, study period 23 January, 2020 - 15 April, 2020, average treatment delay 4.0 days.
Contact: wei_da_chew@ttsh.com.sg (corresponding author).
Clinical Predictors for Abnormal ALT in Patients Infected with COVID-19—A Retrospective Single Centre Study
Pathogens, doi:10.3390/pathogens12030473
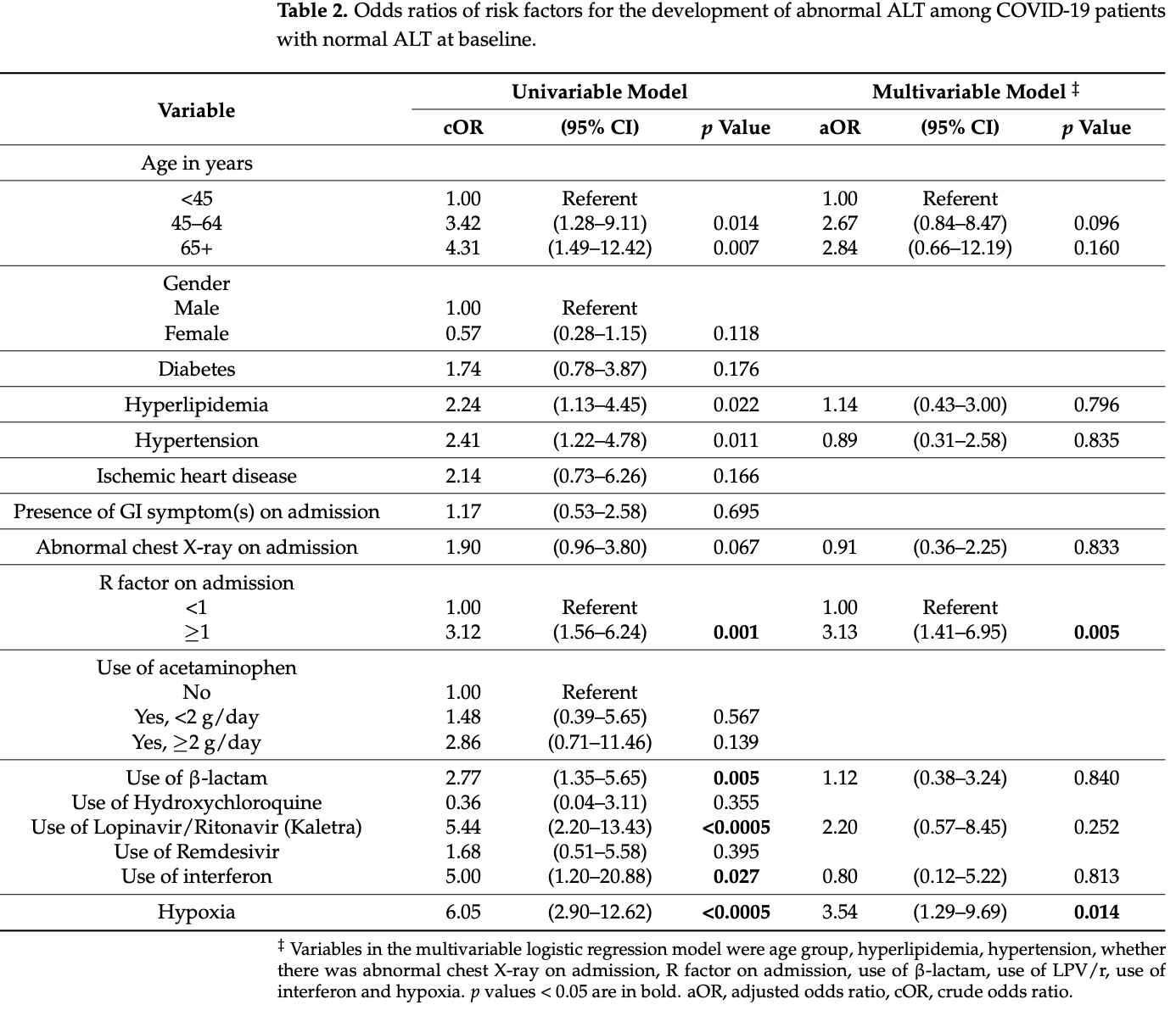
Objective: Abnormal liver tests have been associated with worse clinical outcomes in patients infected with COVID-19. This retrospective observational study from Singapore aims to elucidate simple clinical predictors of abnormal alanine aminotransferase (ALT) in COVID-19 infections. Design: 717 patients hospitalised with COVID-19 at the National Centre for Infectious Diseases (NCID), Singapore, from 23 January-15 April 2020 were screened, of which 163 patients with baseline normal alanine transferase (ALT) and at least two subsequent ALTs performed were included in the final analysis. Information on baseline demographics, clinical characteristics and biochemical laboratory tests were collected. Results: 30.7% of patients developed abnormal ALT. They were more likely to be older (60 vs. 55, p = 0.022) and have comorbidities of hyperlipidaemia and hypertension. The multivariate logistic regression showed that R-factor ≥1 on admission (adjusted odds ratio (aOR) 3.13, 95% Confidence Interval (CI) 1.41-6.95) and hypoxia (aOR 3.54, 95% CI 1.29-9.69) were independent risk factors for developing abnormal ALT. The patients who developed abnormal ALT also ran a more severe course of illness with a greater proportion needing supplementary oxygen (58% vs. 18.6%, p < 0.0005), admission to the Intensive Care Unit (ICU)/High Dependency Unit (HDU) (32% vs. 11.5%, p = 0.003) and intubation (20% vs. 2.7%, p < 0.0005). There was no difference in death rate between the two groups. Conclusions: Liver injury is associated with poor clinical outcomes in patients with COVID-19. R-factor ≥1 on admission and hypoxia are independent simple clinical predictors for developing abnormal ALT in COVID-19.
Conflicts of Interest: Barnaby E Young has received honoraria from Sanofi and Roche outside of the submitted work. Wei Da Chew, Jonathan Kuang, Huiyu Lin, Li Wei Ang, Wei Lyn Yang and David C Lye declare that they have no conflicts of interest.
References
Akdogan, Guzel, Tosun, Akpinar, Diagnostic and early prognostic value of serum CRP and LDH levels in patients with possible COVID-19 at the first admission, J. Infect. Dev. Ctries, doi:10.3855/jidc.14072
Aroniadis, Dimaio, Dixon, Elmunzer, Kolb et al., Current Knowledge and Research Priorities in the Digestive Manifestations of COVID-19, Clin. Gastroenterol. Hepatol, doi:10.1016/j.cgh.2020.04.039
Bertolini, Van De, Peppel, Bodewes, Moshage et al., Abnormal liver function tests in COVID-19 patients: Relevance and potential pathogenesis, Hepatology, doi:10.1002/hep.31480
Cai, Huang, Yu, Zhu, Xia et al., COVID-19: Abnormal liver function tests, J. Hepatol, doi:10.1016/j.jhep.2020.04.006
Dufour, Marjot, Becchetti, Tilg, COVID-19 and liver disease, Gut, doi:10.1136/gutjnl-2021-326792
Fan, Chen, Li, Cheng, Yang et al., Clinical Features of COVID-19-Related Liver Functional Abnormality, Clin. Gastroenterol. Hepatol, doi:10.1016/j.cgh.2020.04.002
Ferm, Fisher, Pakala, Tong, Shah et al., Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, Clin. Gastroenterol. Hepatol, doi:10.1016/j.cgh.2020.05.049
Guan, Ni, Hu, Liang, Qu et al., Clinical characteristics of coronavirus disease 2019 in China, N. Engl. J. Med, doi:10.1056/NEJMoa2002032
Havers, Pham, Taylor, Whitaker, Patel et al., COVID-19-Associated Hospitalizations Among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to, JAMA Intern. Med, doi:10.1001/jamainternmed.2022.4299
Henry, Aggarwal, Wong, Benoit, Vikse et al., Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis, Am. J. Emerg. Med, doi:10.1016/j.ajem.2020.05.073
Hundt, Deng, Ciarleglio, Nathanson, Lim, Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1827 Patients in a Major U.S. Hospital Network, Hepatology, doi:10.1002/hep.31487
Kumar-M, Mishra, Jha, Shukla, Choudhury et al., COVID-19 and the liver: A comprehensive systematic review and meta-analysis, Hepatol. Int, doi:10.1007/s12072-020-10071-9
Kwo, Cohen, Lim, ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries, Am. J. Gastroenterol, doi:10.1038/ajg.2016.517
Lagana, Kudose, Iuga, Lee, Fazlollahi et al., Hepatic pathology in patients dying of COVID-19: A series of 40 cases including clinical, histologic, and virologic data, Mod. Pathol, doi:10.1038/s41379-020-00649-x
Lei, Liu, Zhou, Qin, Zhang et al., Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China, Hepatology, doi:10.1002/hep.31301
Mathieu, Ritchie, Ortiz-Ospina, Roser, Hasell et al., A global database of COVID-19 vaccinations, Nat. Hum. Behav, doi:10.1038/s41562-021-01122-8
Mcgrowder, Miller, Cross, Anderson-Jackson, Bryan et al., Abnormal Liver Biochemistry Tests and Acute Liver Injury in COVID-19 Patients: Current Evidence and Potential Pathogenesis, Diseases, doi:10.3390/diseases9030050
Piano, Dalbeni, Vettore, Benfaremo, Mattioli et al., Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19, Liver Int
Portincasa, Krawczyk, Machill, Lammert, Di Ciaula, Hepatic consequences of COVID-19 infection. Lapping or biting?, Eur. J. Intern. Med, doi:10.1016/j.ejim.2020.05.035
Richardson, Hirsch, Narasimhan, Crawford, Mcginn et al., the Northwell COVID-19 Research Consortium. Presenting Characteristics, Comorbidities and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area, JAMA, doi:10.1001/jama.2020.6775
Sarin, Kumar, Lau, Abbas, Chan et al., Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update, Hepatol. Int, doi:10.1007/s12072-015-9675-4
Sun, Aghemo, Forner, Valenti, COVID-19 and liver disease, Liver Int, doi:10.1111/liv.14470
Torge, Bernardi, Arcangeli, Bianchi, Histopathological Features of SARS-CoV-2 in Extrapulmonary Organ Infection: A Systematic Review of Literature, Pathogens, doi:10.3390/pathogens11080867
Vespa, Pugliese, Piovani, Capogreco, Danese et al., Liver tests abnormalities in COVID-19: Trick or treat?, J. Hepatol, doi:10.1016/j.jhep.2020.05.033
Wang, Gao, Li, Xu, Zhou et al., Early changes in laboratory tests predict liver function damage in patients with moderate coronavirus disease 2019: A retrospective multicenter study, BMC Gastroenterol, doi:10.1186/s12876-022-02188-y
Wang, Yan, Qi, Wu, Zhu et al., Clinical characteristics and risk factors of liver injury in COVID-19: A retrospective cohort study from Wuhan, China, Hepatol. Int, doi:10.1007/s12072-020-10075-5
Wu, Mcgoogan, Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention, JAMA, doi:10.1001/jama.2020.2648
Xu, Shi, Wang, Zhang, Huang et al., Pathological findings of COVID-19 associated with acute respiratory distress syndrome, Lancet Respir. Med, doi:10.1016/S2213-2600(20)30076-X
Yip, Lui, Wong, Chow, Ho et al., Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19, Gut
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.3390/pathogens12030473",
"ISSN": [
"2076-0817"
],
"URL": "http://dx.doi.org/10.3390/pathogens12030473",
"abstract": "<jats:p>Objective: Abnormal liver tests have been associated with worse clinical outcomes in patients infected with COVID-19. This retrospective observational study from Singapore aims to elucidate simple clinical predictors of abnormal alanine aminotransferase (ALT) in COVID-19 infections. Design: 717 patients hospitalised with COVID-19 at the National Centre for Infectious Diseases (NCID), Singapore, from 23 January–15 April 2020 were screened, of which 163 patients with baseline normal alanine transferase (ALT) and at least two subsequent ALTs performed were included in the final analysis. Information on baseline demographics, clinical characteristics and biochemical laboratory tests were collected. Results: 30.7% of patients developed abnormal ALT. They were more likely to be older (60 vs. 55, p = 0.022) and have comorbidities of hyperlipidaemia and hypertension. The multivariate logistic regression showed that R-factor ≥1 on admission (adjusted odds ratio (aOR) 3.13, 95% Confidence Interval (CI) 1.41–6.95) and hypoxia (aOR 3.54, 95% CI 1.29–9.69) were independent risk factors for developing abnormal ALT. The patients who developed abnormal ALT also ran a more severe course of illness with a greater proportion needing supplementary oxygen (58% vs. 18.6%, p < 0.0005), admission to the Intensive Care Unit (ICU)/High Dependency Unit (HDU) (32% vs. 11.5%, p = 0.003) and intubation (20% vs. 2.7%, p < 0.0005). There was no difference in death rate between the two groups. Conclusions: Liver injury is associated with poor clinical outcomes in patients with COVID-19. R-factor ≥1 on admission and hypoxia are independent simple clinical predictors for developing abnormal ALT in COVID-19.</jats:p>",
"alternative-id": [
"pathogens12030473"
],
"author": [
{
"affiliation": [
{
"name": "Department of Gastroenterology & Hepatology, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, Singapore 308433, Singapore"
}
],
"family": "Chew",
"given": "Wei Da",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology & Hepatology, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, Singapore 308433, Singapore"
}
],
"family": "Kuang",
"given": "Jonathan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology & Hepatology, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, Singapore 308433, Singapore"
}
],
"family": "Lin",
"given": "Huiyu",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0036-255X",
"affiliation": [
{
"name": "National Centre for Infectious Diseases, 16 Jalan Tan Tock Seng, Singapore 308442, Singapore"
}
],
"authenticated-orcid": false,
"family": "Ang",
"given": "Li Wei",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Gastroenterology & Hepatology, Tan Tock Seng Hospital, 11 Jalan Tan Tock Seng, Singapore 308433, Singapore"
}
],
"family": "Yang",
"given": "Wei Lyn",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0324-0205",
"affiliation": [
{
"name": "National Centre for Infectious Diseases, 16 Jalan Tan Tock Seng, Singapore 308442, Singapore"
},
{
"name": "Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 636921, Singapore"
}
],
"authenticated-orcid": false,
"family": "Lye",
"given": "David C.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Centre for Infectious Diseases, 16 Jalan Tan Tock Seng, Singapore 308442, Singapore"
},
{
"name": "Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore 636921, Singapore"
}
],
"family": "Young",
"given": "Barnaby E.",
"sequence": "additional"
}
],
"container-title": "Pathogens",
"container-title-short": "Pathogens",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
3,
17
]
],
"date-time": "2023-03-17T06:59:26Z",
"timestamp": 1679036366000
},
"deposited": {
"date-parts": [
[
2023,
3,
17
]
],
"date-time": "2023-03-17T09:46:25Z",
"timestamp": 1679046385000
},
"indexed": {
"date-parts": [
[
2023,
3,
18
]
],
"date-time": "2023-03-18T04:38:55Z",
"timestamp": 1679114335454
},
"is-referenced-by-count": 0,
"issue": "3",
"issued": {
"date-parts": [
[
2023,
3,
16
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2023,
3
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
3,
16
]
],
"date-time": "2023-03-16T00:00:00Z",
"timestamp": 1678924800000
}
}
],
"link": [
{
"URL": "https://www.mdpi.com/2076-0817/12/3/473/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1968",
"original-title": [],
"page": "473",
"prefix": "10.3390",
"published": {
"date-parts": [
[
2023,
3,
16
]
]
},
"published-online": {
"date-parts": [
[
2023,
3,
16
]
]
},
"publisher": "MDPI AG",
"reference": [
{
"key": "ref_1",
"unstructured": "(2023, January 19). WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/."
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "ref_2",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1002/hep.31480",
"article-title": "Abnormal liver function tests in COVID-19 patients: Relevance and potential pathogenesis",
"author": "Bertolini",
"doi-asserted-by": "crossref",
"first-page": "1864",
"journal-title": "Hepatology",
"key": "ref_3",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical characteristics of coronavirus disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N. Engl. J. Med.",
"key": "ref_4",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting Characteristics, Comorbidities and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area",
"author": "Richardson",
"doi-asserted-by": "crossref",
"first-page": "2052",
"journal-title": "JAMA",
"key": "ref_5",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2022.4299",
"article-title": "COVID-19-Associated Hospitalizations Among Vaccinated and Unvaccinated Adults 18 Years or Older in 13 US States, January 2021 to April 2022",
"author": "Havers",
"doi-asserted-by": "crossref",
"first-page": "1071",
"journal-title": "JAMA Intern. Med.",
"key": "ref_6",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1038/s41562-021-01122-8",
"article-title": "A global database of COVID-19 vaccinations",
"author": "Mathieu",
"doi-asserted-by": "crossref",
"first-page": "947",
"journal-title": "Nat. Hum. Behav.",
"key": "ref_7",
"volume": "5",
"year": "2021"
},
{
"DOI": "10.1038/ajg.2016.517",
"article-title": "ACG Clinical Guideline: Evaluation of Abnormal Liver Chemistries",
"author": "Kwo",
"doi-asserted-by": "crossref",
"first-page": "18",
"journal-title": "Am. J. Gastroenterol.",
"key": "ref_8",
"volume": "112",
"year": "2017"
},
{
"DOI": "10.1016/j.jhep.2020.04.006",
"article-title": "COVID-19: Abnormal liver function tests",
"author": "Cai",
"doi-asserted-by": "crossref",
"first-page": "566",
"journal-title": "J. Hepatol.",
"key": "ref_9",
"volume": "13",
"year": "2020"
},
{
"DOI": "10.1002/hep.31487",
"article-title": "Abnormal Liver Tests in COVID-19: A Retrospective Observational Cohort Study of 1827 Patients in a Major U.S. Hospital Network",
"author": "Hundt",
"doi-asserted-by": "crossref",
"first-page": "1169",
"journal-title": "Hepatology",
"key": "ref_10",
"volume": "72",
"year": "2020"
},
{
"DOI": "10.1111/liv.14565",
"article-title": "Abnormal liver function tests predict transfer to intensive care unit and death in COVID-19",
"author": "Piano",
"doi-asserted-by": "crossref",
"first-page": "2394",
"journal-title": "Liver Int.",
"key": "ref_11",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2021-326792",
"article-title": "COVID-19 and liver disease",
"author": "Dufour",
"doi-asserted-by": "crossref",
"first-page": "2350",
"journal-title": "Gut",
"key": "ref_12",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1002/hep.31301",
"article-title": "Longitudinal Association Between Markers of Liver Injury and Mortality in COVID-19 in China",
"author": "Lei",
"doi-asserted-by": "crossref",
"first-page": "389",
"journal-title": "Hepatology",
"key": "ref_13",
"volume": "72",
"year": "2020"
},
{
"key": "ref_14",
"unstructured": "WHO (2020). Clinical Management of COVID 19: Interim Guidance."
},
{
"DOI": "10.1111/liv.14470",
"article-title": "COVID-19 and liver disease",
"author": "Sun",
"doi-asserted-by": "crossref",
"first-page": "1278",
"journal-title": "Liver Int.",
"key": "ref_15",
"volume": "40",
"year": "2020"
},
{
"DOI": "10.1007/s12072-015-9675-4",
"article-title": "Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update",
"author": "Sarin",
"doi-asserted-by": "crossref",
"first-page": "1",
"journal-title": "Hepatol. Int.",
"key": "ref_16",
"volume": "10",
"year": "2016"
},
{
"DOI": "10.1016/j.jhep.2020.05.033",
"article-title": "Liver tests abnormalities in COVID-19: Trick or treat?",
"author": "Vespa",
"doi-asserted-by": "crossref",
"first-page": "1275",
"journal-title": "J. Hepatol.",
"key": "ref_17",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1016/j.cgh.2020.04.039",
"article-title": "Current Knowledge and Research Priorities in the Digestive Manifestations of COVID-19",
"author": "Aroniadis",
"doi-asserted-by": "crossref",
"first-page": "1682",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "ref_18",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/j.cgh.2020.04.002",
"article-title": "Clinical Features of COVID-19-Related Liver Functional Abnormality",
"author": "Fan",
"doi-asserted-by": "crossref",
"first-page": "1561",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "ref_19",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "ref_20",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.cgh.2020.05.049",
"article-title": "Analysis of Gastrointestinal and Hepatic Manifestations of SARS-CoV-2 Infection in 892 Patients in Queens, NY",
"author": "Ferm",
"doi-asserted-by": "crossref",
"first-page": "2378",
"journal-title": "Clin. Gastroenterol. Hepatol.",
"key": "ref_21",
"volume": "18",
"year": "2020"
},
{
"DOI": "10.1007/s12072-020-10075-5",
"article-title": "Clinical characteristics and risk factors of liver injury in COVID-19: A retrospective cohort study from Wuhan, China",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "723",
"journal-title": "Hepatol. Int.",
"key": "ref_22",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1186/s12876-022-02188-y",
"doi-asserted-by": "crossref",
"key": "ref_23",
"unstructured": "Wang, Y., Gao, D., Li, X., Xu, P., Zhou, Q., Yin, J., and Xu, J. (2022). Early changes in laboratory tests predict liver function damage in patients with moderate coronavirus disease 2019: A retrospective multicenter study. BMC Gastroenterol., 22."
},
{
"DOI": "10.20944/preprints202105.0552.v1",
"doi-asserted-by": "crossref",
"key": "ref_24",
"unstructured": "McGrowder, D., Miller, F., Cross, M.A., Anderson-Jackson, L., Bryan, S., and Dilworth, L. (2021). Abnormal Liver Biochemistry Tests and Acute Liver Injury in COVID-19 Patients: Current Evidence and Potential Pathogenesis. Diseases, 9."
},
{
"DOI": "10.1007/s12072-020-10071-9",
"article-title": "COVID-19 and the liver: A comprehensive systematic review and meta-analysis",
"author": "Mishra",
"doi-asserted-by": "crossref",
"first-page": "711",
"journal-title": "Hepatol. Int.",
"key": "ref_25",
"volume": "14",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.05.035",
"article-title": "Hepatic consequences of COVID-19 infection. Lapping or biting?",
"author": "Portincasa",
"doi-asserted-by": "crossref",
"first-page": "18",
"journal-title": "Eur. J. Intern. Med.",
"key": "ref_26",
"volume": "77",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-321726",
"article-title": "Liver injury is independently associated with adverse clinical outcomes in patients with COVID-19",
"author": "Yip",
"doi-asserted-by": "crossref",
"first-page": "733",
"journal-title": "Gut",
"key": "ref_27",
"volume": "70",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30076-X",
"article-title": "Pathological findings of COVID-19 associated with acute respiratory distress syndrome",
"author": "Xu",
"doi-asserted-by": "crossref",
"first-page": "420",
"journal-title": "Lancet Respir. Med.",
"key": "ref_28",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1038/s41379-020-00649-x",
"article-title": "Hepatic pathology in patients dying of COVID-19: A series of 40 cases including clinical, histologic, and virologic data",
"author": "Lagana",
"doi-asserted-by": "crossref",
"first-page": "2147",
"journal-title": "Mod. Pathol.",
"key": "ref_29",
"volume": "33",
"year": "2020"
},
{
"DOI": "10.1016/j.ajem.2020.05.073",
"article-title": "Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: A pooled analysis",
"author": "Henry",
"doi-asserted-by": "crossref",
"first-page": "1722",
"journal-title": "Am. J. Emerg. Med.",
"key": "ref_30",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.3390/pathogens11080867",
"doi-asserted-by": "crossref",
"key": "ref_31",
"unstructured": "Torge, D., Bernardi, S., Arcangeli, M., and Bianchi, S. (2022). Histopathological Features of SARS-CoV-2 in Extrapulmonary Organ Infection: A Systematic Review of Literature. Pathogens, 11."
},
{
"DOI": "10.3855/jidc.14072",
"article-title": "Diagnostic and early prognostic value of serum CRP and LDH levels in patients with possible COVID-19 at the first admission",
"author": "Akdogan",
"doi-asserted-by": "crossref",
"first-page": "766",
"journal-title": "J. Infect. Dev. Ctries.",
"key": "ref_32",
"volume": "15",
"year": "2021"
}
],
"reference-count": 32,
"references-count": 32,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.mdpi.com/2076-0817/12/3/473"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"General Immunology and Microbiology",
"Molecular Biology",
"Immunology and Allergy"
],
"subtitle": [],
"title": "Clinical Predictors for Abnormal ALT in Patients Infected with COVID-19—A Retrospective Single Centre Study",
"type": "journal-article",
"volume": "12"
}
chew2