DrugWAS: Drug‐wide Association Studies for COVID‐19 Drug Repurposing
et al., Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2376, Feb 2021 (preprint)
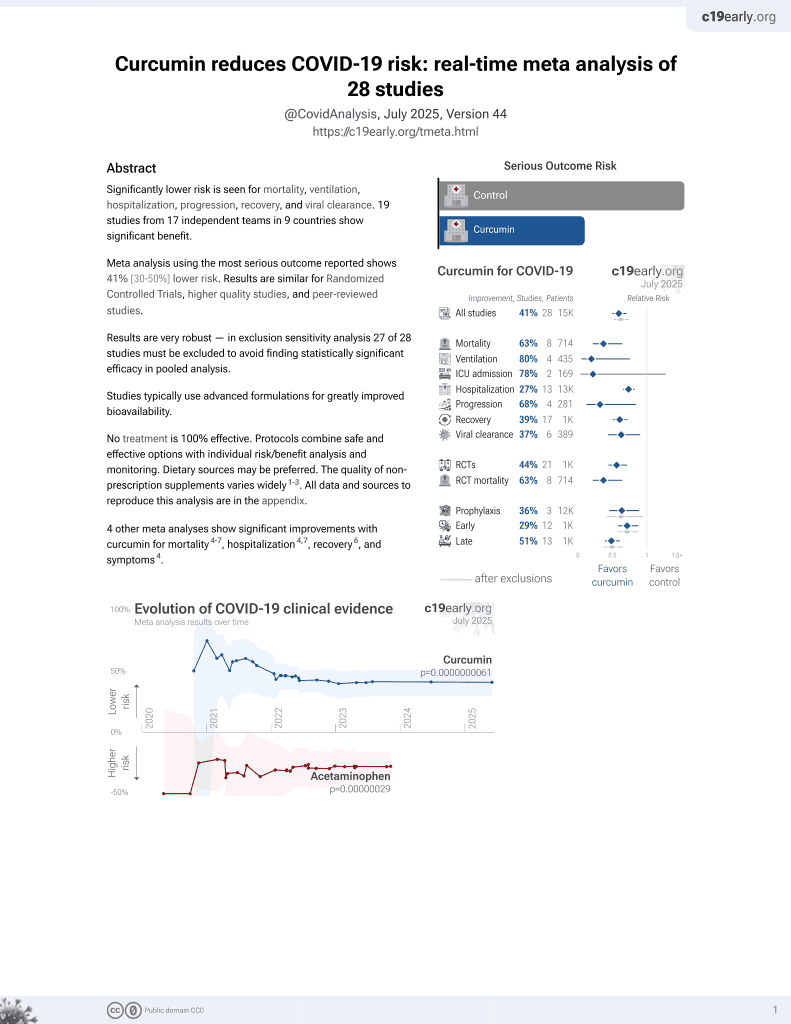
Curcumin for COVID-19
17th treatment shown to reduce risk in
February 2021, now with p = 0.0000000061 from 28 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
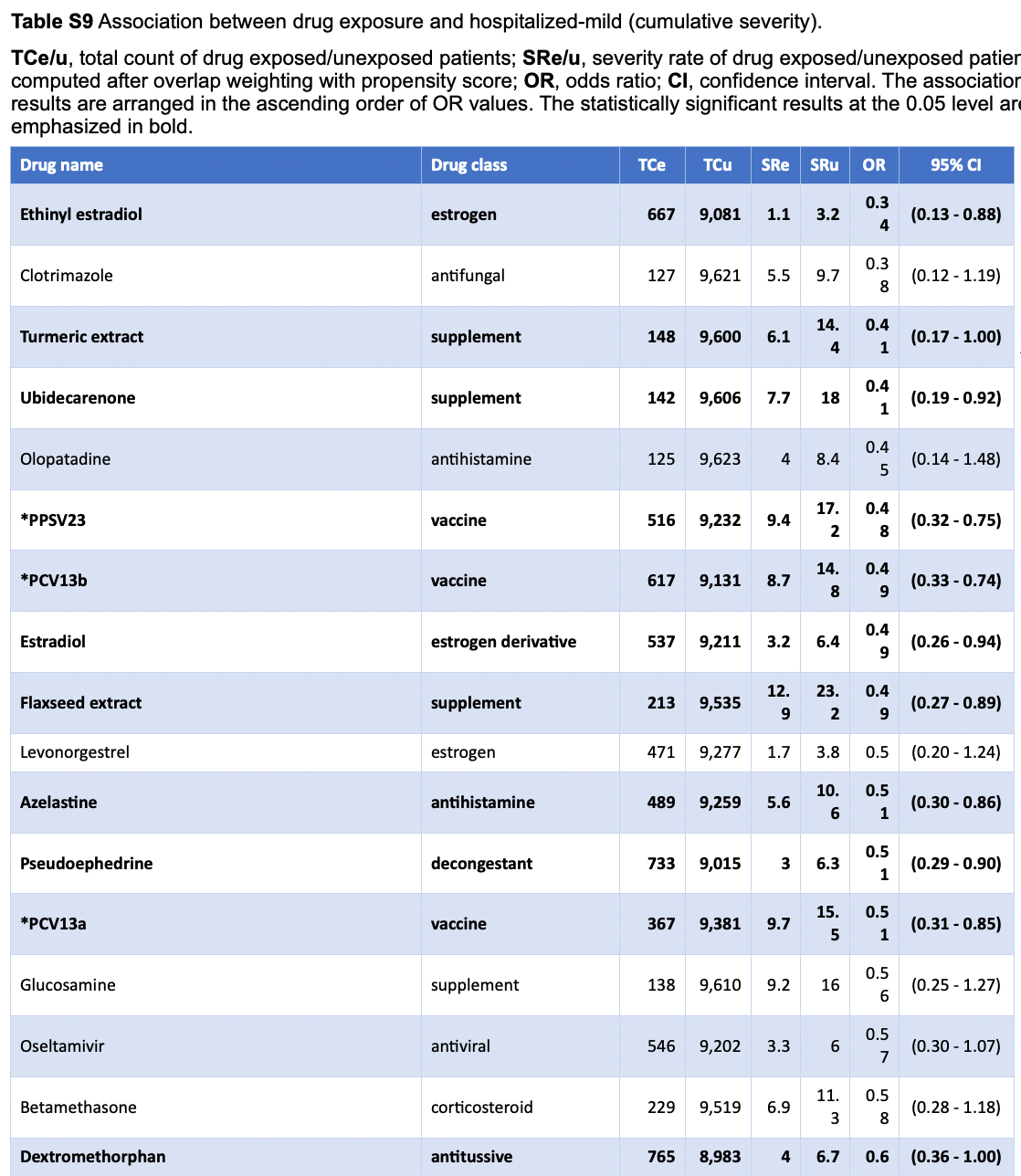
Retrospective 9,748 COVID-19 patients in the USA showing lower hospitalization with turmeric extract.
This is the 3rd of 28 COVID-19 controlled studies for curcumin, which collectively show efficacy with p=0.0000000061.
21 studies are RCTs, which show efficacy with p=0.0000022.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of hospitalization, 59.0% lower, OR 0.41, p = 0.048, treatment 148, control 9,600, adjusted per study, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Bejan et al., 28 Feb 2021, retrospective, USA, peer-reviewed, mean age 42.0, 6 authors.
Contact: adi.bejan@vanderbilt.edu.
DrugWAS: Drug‐wide Association Studies for COVID‐19 Drug Repurposing
Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2376
Study Highlights WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC? Drug repurposing methodologies have emerged as an attractive strategy to rapidly identify safe and effective treatments for coronavirus disease 2019 (COVID-19). Despite recent advances, widely available treatments that can be used early in a patient's illness to prevent hospitalization, progression to more severe outcomes, and long-term complications have yet to be discovered. WHAT QUESTION DID THIS STUDY ADDRESS? Can electronic health records be used to search for drug candidates that could be repurposed to treat COVID-19? WHAT DOES THIS STUDY ADD TO OUR KNOW-LEDGE? The study found 17 drug ingredients that are significantly associated with a decreased risk of death and other severe COVID-19 outcomes. The study suggests that Streptococcus pneumoniae vaccines and diphtheria toxoid and tetanus toxoid vaccine, with or without acellular pertussis vaccine should not be delayed or discontinued due to the COVID-19 pandemic as they may protect the general population from severe acute respiratory syndrome coronavirus 2 infection worldwide.
HOW MIGHT THIS CHANGE CLINICAL PHARMA-COLOGY OR TRANSLATIONAL SCIENCE? The list of drugs proposed by this study could provide additional insights into developing new candidates for COVID-19 treatment.
SUPPORTING INFORMATION Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
CONFLICT OF INTEREST K.N.C. reported personal fees from Teva, personal fees from Optinose, personal fees from Novartis, personal fees from GlaxoSmithKline, personal fees from Blueprint Medicines, personal fees from Third Harmonic Bio, personal fees from Sanofi Pasteur, personal fees from Genentech, personal fees from Regeneron and personal fees from Ribon Therapeutics, outside the submitted work. J.F.P. reported personal fees from Color Genomics outside the submitted work. E.J.P. receives Royalties from UpToDate and consulting fees from Janssen, Vertex, Biocryst, and Regeneron outside of the submitted work. She is co-director of IIID Pty Ltd that holds a patent for HLA-B*57:01 testing for abacavir hypersensitivity, and has a patent pending for Detection of Human Leukocyte Antigen-A*32:01 in connection with Diagnosing Drug Reaction with Eosinophilia and Systemic Symptoms without any financial remuneration and not directly related to the submitted work. All other authors declared no competing interests for this work.
AUTHOR CONTRIBUTIONS
ROLE OF THE FUNDER/SPONSOR The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
Al-Ani, Review article: prevention, diagnosis and management of COVID-19 in the IBD patient, Aliment. Pharmacol. Ther
Asher, Tintle, Myers, Lockshon, Bacareza et al., Blood omega-3 fatty acids and death from COVID-19: A pilot study, Prostaglandins Leukot. Essent. Fatty Acids
Baden, Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine, N. Engl. J. Med
Beigel, Remdesivir for the treatment of covid-19 -final report, N. Engl. J. Med
Bejan, Cahill, Staso, Choi, Peterson et al., Leveraging drug-wide association studies to facilitate drug repurposing for COVID-19, doi:10.1101/2021.02.04.21251169
Bejan, Wei, Denny, Assessing the role of a medication-indication resource in the treatment relation extraction from clinical text, J. Am. Med. Inform. Assoc
Castro, Ross, Mcbride, Perlis, Identifying common pharmacotherapies associated with reduced COVID-19 morbidity using electronic health records, doi:10.1101/2020.04.11.20061994
Cava, Bertoli, Castiglioni, In silico discovery of candidate drugs against COVID-19, Viruses
Chen, SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with COVID-19, N. Engl. J. Med
Choi, Development of a system for postmarketing population pharmacokinetic and pharmacodynamic studies using real-world data from electronic health records, Clin. Pharmacol. Therap
D'agostino, Jr & D'agostino, Sr, Estimating treatment effects using observational data, JAMA
Day, Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists, BMJ
De Faria Coelho-Ravagnani, Corgosinho, Sanches, Prado, Laviano et al., Dietary recommendations during the COVID-19 pandemic, Nutr. Rev
Delozier, Phenotyping coronavirus disease 2019 during a global health pandemic: lessons learned from the characterization of an early cohort, J. Biomed. Inform
Elixhauser, Steiner, Harris, Coffey, Comorbidity measures for use with administrative data, Med. Care
Gordon, A SARS-CoV-2 protein interaction map reveals targets for drug repurposing, Nature
Gupta, Factors associated with death in critically Ill patients with coronavirus disease 2019 in the US, JAMA Intern Med
Hoertel, Association between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID-19: an observational multicenter study, Clin. Pharmacol. Ther, doi:10.1002/cpt.2317
Hoertel, Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study, Mol Psychiatry, doi:10.1038/s41380-021-01021-4
Ietto, SARS -CoV-2: Reasons of epidemiology of severe ill disease cases and therapeutic approach using trivalent vaccine (tetanus, diphtheria and Bordetella pertussis), Med. Hypotheses
Kim, Read, Fauci, Therapy for early COVID-19: A critical need, JAMA
Konrat, The anti-histamine azelastine, identified by computational drug repurposing, inhibits SARS-CoV-2 infection in reconstituted human nasal tissue in vitro, doi:10.1101/2020.09.15.296228
Kragholm, Association between prescribed ibuprofen and severe COVID-19 infection: A nationwide register-based cohort study, Clin. Transl. Sci
Lenze, Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: A randomized clinical trial, JAMA
Lewnard, Prevention of COVID-19 among older adults receiving pneumococcal conjugate vaccine suggests interactions between Streptococcus pneumoniae and SARS-CoV-2 in the respiratory tract, J. Infect. Dis, doi:10.1093/infdis/jiab128
Li, Network bioinformatics analysis provides insight into drug repurposing for COVID-2019, Med. Drug Discov, doi:10.1016/j.medidd.2021.100090
Li, Thomas, Li, Addressing extreme propensity scores via the overlap weights, Am. J. Epidemiol
Liao, Petrache, Fingerlin, Maier, Association of inhaled and systemic corticosteroid use with Coronavirus Disease 2019 (COVID-19) test positivity in patients with chronic pulmonary diseases, Respir Med
Manoharan, Haridas, Vasanthakumar, Muthu, Thavoorullah et al., Curcumin: a Wonder Drug as a Preventive Measure for COVID19 Management, Indian J. Clin. Bioche
Matsuyama, The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replicationtranscription complex in cultured cells, J Virol
Mehta, Association of use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19), JAMA Cardiol
Nicola, The socio-economic implications of the coronavirus pandemic (COVID-19): A review, Int. J. Surg
Nunes, Cutland, Klugman, Madhi, Pneumococcal conjugate vaccine protection against coronavirusassociated pneumonia hospitalization in children living with and without HIV, mBio, doi:10.1128/mBio.02347-20
Paules, Marston, Fauci, Coronavirus infectionsmore than just the common cold, JAMA
Polack, Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine, N. Engl. J. Med
Quan, Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data, Med, Care
Reche, Potential cross-reactive immunity to SARS-CoV-2 from common human pathogens and vaccines, Front. Immunol
Reznikov, Identification of antiviral antihistamines for COVID-19 repurposing, Biochem. Biophys. Res. Commun
Rinott, Kozer, Shapira, Bar-Haim, Youngster, Ibuprofen use and clinical outcomes in COVID-19 patients, Clin. Microbiol. Infec
Riva, Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing, Nature
Rubin, Study aims to identify drugs that could be repurposed for COVID-19, JAMA
Sanders, Monogue, Jodlowski, Cutrell, Pharmacologic treatments for coronavirus disease 2019 (COVID-19): A review, JAMA
Seeland, Evidence for treatment with estradiol for women with SARS-CoV-2 infection, BMC Med
Sender, Hentrich, Henriques-Normark, Virus-induced changes of the respiratory tract environment promote secondary infections with streptococcus pneumoniae, Front. Cell. Infect. Microbiol
Sohn, Clark, Halgrim, Murphy, Chute et al., MedXN: an open source medication extraction and normalization tool for clinical text, J. Am. Med. Inform. Assoc
Thomas, Li, Pencina, Overlap weighting a propensity score method that mimics attributes of a randomized clinical trial, JAMA
Weeks, medExtractR: A targeted, customizable approach to medication extraction from electronic health records, J. Am. Med. Inform. Assoc
Wiersinga, Rhodes, Cheng, Peacock, Prescott, Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A Review, JAMA
Zhou, Hou, Shen, Huang, Martin et al., Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2
DOI record:
{
"DOI": "10.1002/cpt.2376",
"ISSN": [
"0009-9236",
"1532-6535"
],
"URL": "http://dx.doi.org/10.1002/cpt.2376",
"alternative-id": [
"10.1002/cpt.2376"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-04-22"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2021-07-21"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2021-08-10"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Biomedical Informatics Vanderbilt University Medical Center Nashville Tennessee USA"
}
],
"family": "Bejan",
"given": "Cosmin A.",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Medicine Division of Allergy, Pulmonary and Critical Care Medicine Vanderbilt University Medical Center Nashville Tennessee USA"
}
],
"family": "Cahill",
"given": "Katherine N.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine Division of Allergy, Pulmonary and Critical Care Medicine Vanderbilt University Medical Center Nashville Tennessee USA"
}
],
"family": "Staso",
"given": "Patrick J.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biostatistics Vanderbilt University Medical Center Nashville Tennessee USA"
}
],
"family": "Choi",
"given": "Leena",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Biomedical Informatics Vanderbilt University Medical Center Nashville Tennessee USA"
},
{
"name": "Department of Medicine Vanderbilt University Medical Center Nashville Tennessee USA"
}
],
"family": "Peterson",
"given": "Josh F.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pathology, Microbiology and Immunology Vanderbilt University Medical Center Nashville Tennessee USA"
},
{
"name": "Department of Medicine Division of Infectious Diseases Vanderbilt University Medical Center Nashville Tennessee USA"
},
{
"name": "Department of Pharmacology Vanderbilt University Medical Center Nashville Tennessee USA"
}
],
"family": "Phillips",
"given": "Elizabeth J.",
"sequence": "additional"
}
],
"container-title": "Clinical Pharmacology & Therapeutics",
"container-title-short": "Clin Pharma and Therapeutics",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2021,
7,
27
]
],
"date-time": "2021-07-27T17:06:28Z",
"timestamp": 1627405588000
},
"deposited": {
"date-parts": [
[
2022,
4,
20
]
],
"date-time": "2022-04-20T07:14:11Z",
"timestamp": 1650438851000
},
"funder": [
{
"DOI": "10.13039/100000002",
"award": [
"P50GM115305",
"R01HG010863",
"R01AI150295",
"K23AI118804",
"R01GM124109",
"UL1TR000445"
],
"doi-asserted-by": "publisher",
"name": "National Institutes of Health"
}
],
"indexed": {
"date-parts": [
[
2023,
7,
20
]
],
"date-time": "2023-07-20T22:50:27Z",
"timestamp": 1689893427358
},
"is-referenced-by-count": 10,
"issue": "6",
"issued": {
"date-parts": [
[
2021,
8,
10
]
]
},
"journal-issue": {
"issue": "6",
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
10
]
],
"date-time": "2021-08-10T00:00:00Z",
"timestamp": 1628553600000
}
},
{
"URL": "http://doi.wiley.com/10.1002/tdm_license_1.1",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
8,
10
]
],
"date-time": "2021-08-10T00:00:00Z",
"timestamp": 1628553600000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.2376",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/cpt.2376",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/cpt.2376",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1537-1546",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2021,
8,
10
]
]
},
"published-online": {
"date-parts": [
[
2021,
8,
10
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1001/jama.2020.0757",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_1_1"
},
{
"DOI": "10.1016/j.ijsu.2020.04.018",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_2_1"
},
{
"DOI": "10.1056/NEJMoa2034577",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_3_1"
},
{
"DOI": "10.1056/NEJMoa2035389",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1101/2020.06.22.20137273",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_6_1",
"unstructured": "[No authors listed].Dexamethasone in hospitalized patients with COVID‐19 ‐ preliminary reporthttps://doi.org/10.1101/2020.06.22.20137273"
},
{
"DOI": "10.1056/NEJMoa2029849",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_7_1"
},
{
"DOI": "10.1001/jama.2019.20153",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.1001/jama.2020.12839",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1001/jama.2020.22760",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1001/jama.2020.22813",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1038/s41586-020-2577-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_12_1"
},
{
"DOI": "10.1038/s41421-020-0153-3",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_13_1"
},
{
"DOI": "10.1136/amiajnl-2014-002954",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_14_1"
},
{
"DOI": "10.1136/amiajnl-2013-002190",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_15_1"
},
{
"DOI": "10.1093/jamia/ocz207",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_16_1"
},
{
"DOI": "10.1002/cpt.1787",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_17_1"
},
{
"DOI": "10.1001/jamainternmed.2020.3596",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1097/00005650-199801000-00004",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1097/01.mlr.0000182534.19832.83",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1001/jama.297.3.314",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1001/jama.2020.7819",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"article-title": "Addressing extreme propensity scores via the overlap weights",
"author": "Li F.",
"first-page": "250",
"journal-title": "Am. J. Epidemiol.",
"key": "e_1_2_11_23_1",
"volume": "188",
"year": "2019"
},
{
"DOI": "10.1001/jamacardio.2020.1855",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1016/j.rmed.2020.106275",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.1016/j.bbrc.2020.11.095",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"article-title": "Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID‐19: results from an observational study",
"author": "Hoertel N.",
"first-page": "1",
"journal-title": "Mol Psychiatry",
"key": "e_1_2_11_27_1",
"year": "2021"
},
{
"article-title": "Association between FIASMAs and Reduced Risk of Intubation or Death in Individuals Hospitalized for Severe COVID‐19: an observational multicenter study",
"author": "Hoertel N.",
"journal-title": "Clin. Pharmacol. Ther",
"key": "e_1_2_11_28_1"
},
{
"DOI": "10.1001/jama.2020.21726",
"article-title": "Study aims to identify drugs that could be repurposed for COVID‐19",
"author": "Rubin R.",
"doi-asserted-by": "crossref",
"first-page": "2019",
"journal-title": "JAMA",
"key": "e_1_2_11_29_1",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1186/s12916-020-01851-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_30_1"
},
{
"DOI": "10.1101/2020.09.15.296228",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_31_1",
"unstructured": "Konrat R.et al.The anti‐histamine azelastine identified by computational drug repurposing inhibits SARS‐CoV‐2 infection in reconstituted human nasal tissue in vitro. bioRxivhttps://doi.org/10.1101/2020.09.15.296228."
},
{
"article-title": "Network bioinformatics analysis provides insight into drug repurposing for COVID‐2019",
"author": "Li X.",
"journal-title": "Med. Drug Discov",
"key": "e_1_2_11_32_1"
},
{
"DOI": "10.1038/s41586-020-2286-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_33_1"
},
{
"DOI": "10.1016/j.plefa.2021.102250",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_34_1"
},
{
"DOI": "10.3390/v12040404",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_35_1"
},
{
"DOI": "10.1128/JVI.01648-20",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_36_1"
},
{
"DOI": "10.1136/bmj.m1086",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_37_1"
},
{
"DOI": "10.1016/j.cmi.2020.06.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_38_1"
},
{
"DOI": "10.1111/cts.12904",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_39_1"
},
{
"DOI": "10.1101/2020.04.11.20061994",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_40_1",
"unstructured": "Castro V.M. Ross R.A. McBride S.&Perlis R.H.Identifying common pharmacotherapies associated with reduced COVID‐19 morbidity using electronic health records. medRxivhttps://doi.org/10.1101/2020.04.11.20061994"
},
{
"DOI": "10.1101/2021.02.04.21251169",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_41_1",
"unstructured": "Bejan C.A. Cahill K.N. Staso P.J. Choi L. Peterson J.F.&Phillips E.J.DrugWAS: Leveraging drug‐wide association studies to facilitate drug repurposing for COVID‐19. medRxivhttps://doi.org/10.1101/2021.02.04.21251169"
},
{
"article-title": "Prevention of COVID‐19 among older adults receiving pneumococcal conjugate vaccine suggests interactions between Streptococcus pneumoniae and SARS‐CoV‐2 in the respiratory tract",
"author": "Lewnard J.A.",
"journal-title": "J. Infect. Dis",
"key": "e_1_2_11_42_1"
},
{
"article-title": "Pneumococcal conjugate vaccine protection against coronavirus‐associated pneumonia hospitalization in children living with and without HIV",
"author": "Nunes M.C.",
"journal-title": "mBio",
"key": "e_1_2_11_43_1",
"volume": "12"
},
{
"DOI": "10.1111/apt.15779",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_44_1"
},
{
"DOI": "10.3389/fcimb.2021.643326",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_45_1"
},
{
"DOI": "10.3389/fimmu.2020.586984",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_46_1"
},
{
"DOI": "10.1016/j.mehy.2020.109779",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_47_1"
},
{
"DOI": "10.1093/nutrit/nuaa067",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_48_1"
},
{
"DOI": "10.1007/s12291-020-00902-9",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_49_1"
},
{
"DOI": "10.1016/j.jbi.2021.103777",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_50_1"
}
],
"reference-count": 50,
"references-count": 50,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/cpt.2376"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Pharmacology"
],
"subtitle": [],
"title": "DrugWAS: Drug‐wide Association Studies for COVID‐19 Drug Repurposing",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "110"
}
bejan