Pentoxifylline effects on hospitalized patients with COVID19: A randomized, double-blind clinical trial
et al., International Immunopharmacology, doi:10.1016/j.intimp.2021.108227, IRCT20190804044429N4, Dec 2021
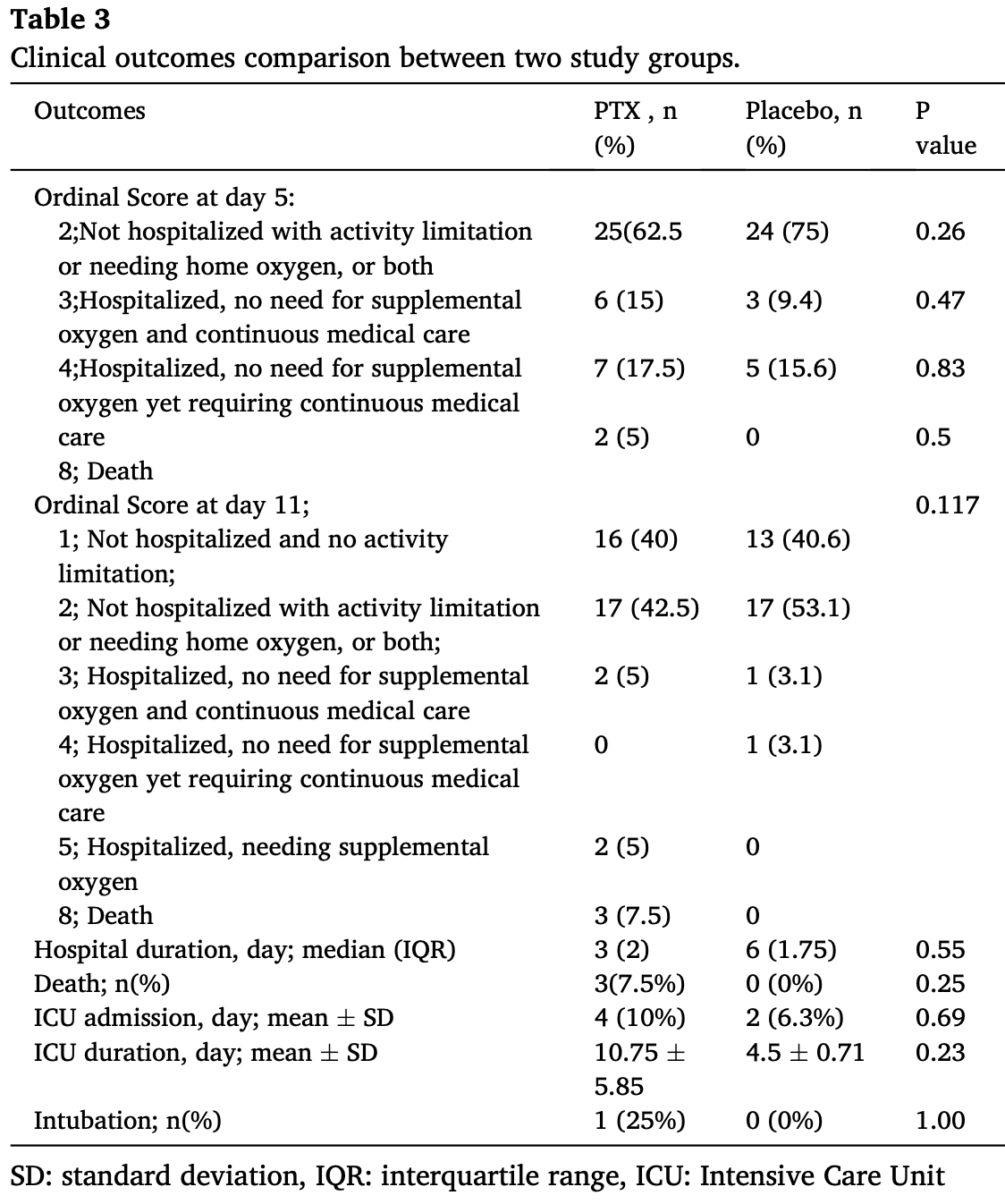
RCT 72 hospitalized COVID-19 patients showing no significant difference in clinical outcomes with pentoxifylline treatment compared to placebo.
|
risk of death, 540.0% higher, RR 6.40, p = 0.25, treatment 3 of 40 (7.5%), control 0 of 32 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 60.0% higher, RR 1.60, p = 1.00, treatment 2 of 40 (5.0%), control 1 of 32 (3.1%).
|
|
risk of ICU admission, 60.0% higher, RR 1.60, p = 0.69, treatment 4 of 40 (10.0%), control 2 of 32 (6.2%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Azizi et al., 31 Dec 2021, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, mean age 58.9, 8 authors, study period November 2020 - April 2021, average treatment delay 5.75 days, trial IRCT20190804044429N4.
Contact: ghazaeianm@gmail.com.
Pentoxifylline effects on hospitalized patients with COVID19: A randomized, double-blind clinical trial
International Immunopharmacology, doi:10.1016/j.intimp.2021.108227
A B S T R A C T Pentoxifylline (PTX) has broad-spectrum properties such as anti-inflammatory, anticoagulant, and antiviral effects. The aim of this study was to evaluate the efficacy and safety of PTX in hospitalized patients with COVID-19. This double-blind, placebo-controlled randomized clinical trial was conducted on hospitalized patients with COVID-19. The recruited patients were randomly (1:1) assigned to the PTX group and the placebo group. The intervention group received PTX capsules at a dose of 400 mg three times a day for 10 days along with the national regimen, including interferon plus lopinavir/ritonavir and hydroxychloroquine. The primary outcome was the improvement of clinical scores. The secondary outcomes, on the other hand, were improvement in inflammatory and oxidative stress factors and hospital complications. From a total of 102 patients who met the inclusion criteria, 72 individuals completed the study and were analyzed. No significant differences were shown in demographics and baseline clinical characteristics. Clinical scores was not significant between the two groups (P = 0.31 and 0.07 for day 5 and 11, respectively). Although the mean serum levels of interleukin-6 (IL-6) and glutathione changed significantly after 5 days in the PTX group (P = 0.03 and p = 0.04), ICU admission, intubation, and hospital stay did not differ between the two groups. The results of our study did not show any superiority of PTX over placebo in improving the clinical outcomes of patients with COVID-19. Although PTX had a beneficial effect on IL-6 and showed an acceptable safety profile, it did not offer any clinical benefit for COVID-19 complications.
Ethics approval This study follows the declaration of Helsinki and was approved by the Ethics Committee of Mazandaran University of Medical Sciences (IR. MAZUMS.REC.1399.744).
CRediT authorship contribution statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Adel, Awad, Abdel-Naim, Al-Azizi, Effects of pentoxifylline on coagulation profile and disseminated intravascular coagulation incidence in Egyptian septic neonates, J. Clin. Pharm. Ther
Amvros' Eva, Votiakov, Andreeva, Vladyko, Nikolaeva et al., New properties of trental as an inhibitor of viral activity with a wide range of activity, Voprosy virusologii
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19, N. Engl. J. Med
Cao, COVID-19: immunopathology and its implications for therapy, Nat. Rev. Immunol
Chavarría, Vázquez, Cherit, Bello, Suastegui et al., Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19, Comput. Struct. Biotechnol. J
De, Gangopadhyay, Dutta, Baksi, Pani et al., Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial, World journal of gastroenterology: WJG, doi:10.3748/wjg.15.1613
Fazely, Dezube, Allen-Ryan, Pardee, Ruprecht, Pentoxifylline (Trental) decreases the replication of the human immunodeficiency virus type 1 in human peripheral blood mononuclear cells and in cultured T cells
Ghasemnejad-Berenji, Pashapour, Sadeghpour, Pentoxifylline: A Drug with Antiviral and Anti-Inflammatory Effects to Be Considered in the Treatment of Coronavirus Disease, Medical Principles and Practice
Gupta, Johnson, Mather, Clauss, Rehman et al., Anti-inflammatory treatment with pentoxifylline improves HIV-related endothelial dysfunction: a pilot study, AIDS
Harris, Schulzke, Patole, Pentoxifylline in preterm neonates, Pediatric Drugs
Jiménez-Luévano, Lerma-Díaz, Hernández-Flores, Jiménez-Partida, Bravo-Cuellar, Addition of pentoxifylline to pegylated interferon-alpha-2a and ribavirin improves sustained virological response to chronic hepatitis C virus: a randomized clinical trial, Annals of hepatology
Konrad, Neudeck, Vollmer, Ngamsri, Thiel et al., Protective effects of pentoxifylline in pulmonary inflammation are adenosine receptor A2A dependent, FASEB J
Li, Zuo, Tang, Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases, Front. Pharmacol
Lissoni, Ardizzoia, Barni, Pittalis, Rossini et al., Characterization of cancer-related disseminated intravascular coagulation in relation to tumor necrosis factor-alpha blood concentrations: Possible therapeutic role of pentoxifylline, Tumori Journal
Maldonado, Hernandez-Ramírez, Oliva-Pérez, Sánchez-Martínez, Pimentel-González et al., Pentoxifylline decreases serum LDH levels and increases lymphocyte count in COVID-19 patients: results from an external pilot study, International Immunopharmacology, doi:10.1016/j.intimp.2020.107209
Maldonado, Loza-Mejía, Chávez-Alderete, Repositioning of pentoxifylline as an immunomodulator and regulator of the renin-angiotensin system in the treatment of COVID-19, Med. Hypotheses, doi:10.1016/j.mehy.2020.109988
Martín, Jiménez, Mueóz-Fernández, Pentoxifylline and severe acute respiratory syndrome (SARS): a drug to be considered, Med. Sci. Monit
Neuner, Klosner, Schauer, Pourmojib, Macheiner et al., Pentoxifylline in vivo down-regulates the release of IL-1 beta, IL-6, IL-8 and tumour necrosis factor-alpha by human peripheral blood mononuclear cells, Immunology
Okunieff, Augustine, Hicks, Cornelison, Altemus et al., Pentoxifylline in the treatment of radiation-induced fibrosis, J. Clin. Oncol
Organization, Clinical management of COVID-19: interim guidance
Organization, SARI) when COVID-19 disease is suspected: interim guidance, 13
Paules, Marston, Fauci, Coronavirus infections-more than just the common cold, JAMA
Rubin, Chan-Tack, Farley, Sherwat, FDA approval of remdesivir-a step in the right direction, N. Engl. J. Med
Schulzke, Kaempfen, Patole, Pentoxifylline for the prevention of bronchopulmonary dysplasia in preterm infants, Cochrane Database of Systematic Reviews
Wall, Smith, Trump, Mohr, Dumontier et al., Pentoxifylline or theophylline use in hospitalized COVID-19 patients requiring oxygen support, The Clinical Respiratory Journal
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Ye, Wang, Mao, The pathogenesis and treatment of theCytokine Storm'in COVID-19, J. Infect
Zhu, Zhang, Wang, Li, Yang et al., A novel coronavirus from patients with pneumonia in China, N. Engl. J. Med
DOI record:
{
"DOI": "10.1016/j.intimp.2021.108227",
"ISSN": [
"1567-5769"
],
"URL": "http://dx.doi.org/10.1016/j.intimp.2021.108227",
"alternative-id": [
"S1567576921008638"
],
"article-number": "108227",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Pentoxifylline effects on hospitalized patients with COVID19: A randomized, double-blind clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Immunopharmacology"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.intimp.2021.108227"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier B.V. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Azizi",
"given": "Hanieh",
"sequence": "first"
},
{
"affiliation": [],
"family": "Rouhani",
"given": "Nima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaki",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Karimpour-razkenari",
"given": "Elahe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghazaeian",
"given": "Monireh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salehifar",
"given": "Ebrahim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saeedi",
"given": "Majid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fallah",
"given": "Sahar",
"sequence": "additional"
}
],
"container-title": "International Immunopharmacology",
"container-title-short": "International Immunopharmacology",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
8
]
],
"date-time": "2021-10-08T03:35:34Z",
"timestamp": 1633664134000
},
"deposited": {
"date-parts": [
[
2023,
12,
3
]
],
"date-time": "2023-12-03T16:18:31Z",
"timestamp": 1701620311000
},
"funder": [
{
"DOI": "10.13039/501100004160",
"award": [
"IRMAZUMS8294"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004160",
"id-type": "DOI"
}
],
"name": "Mazandaran University of Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2025,
2,
21
]
],
"date-time": "2025-02-21T04:40:44Z",
"timestamp": 1740112844401,
"version": "3.37.3"
},
"is-referenced-by-count": 4,
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
12,
1
]
],
"date-time": "2021-12-01T00:00:00Z",
"timestamp": 1638316800000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S1567576921008638?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S1567576921008638?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "108227",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1001/jama.2020.0757",
"article-title": "Coronavirus infections—more than just the common cold",
"author": "Paules",
"doi-asserted-by": "crossref",
"first-page": "707",
"issue": "8",
"journal-title": "JAMA.",
"key": "10.1016/j.intimp.2021.108227_b0005",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "crossref",
"first-page": "727",
"issue": "8",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.intimp.2021.108227_b0010",
"volume": "382",
"year": "2020"
},
{
"key": "10.1016/j.intimp.2021.108227_b0015",
"unstructured": "World Health Organization coronavirus disease 2019 (Covid-19) situation report. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 19 February 2020)."
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"issue": "13",
"journal-title": "JAMA.",
"key": "10.1016/j.intimp.2021.108227_b0020",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/j.jinf.2020.03.037",
"article-title": "The pathogenesis and treatment of theCytokine Storm'in COVID-19",
"author": "Ye",
"doi-asserted-by": "crossref",
"first-page": "607",
"issue": "6",
"journal-title": "J. Infect.",
"key": "10.1016/j.intimp.2021.108227_b0025",
"volume": "80",
"year": "2020"
},
{
"DOI": "10.1038/s41577-020-0308-3",
"article-title": "COVID-19: immunopathology and its implications for therapy",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "269",
"issue": "5",
"journal-title": "Nat. Rev. Immunol.",
"key": "10.1016/j.intimp.2021.108227_b0030",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1056/NEJMp2032369",
"article-title": "FDA approval of remdesivir—a step in the right direction",
"author": "Rubin",
"doi-asserted-by": "crossref",
"first-page": "2598",
"issue": "27",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.intimp.2021.108227_b0035",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1159/000512234",
"article-title": "Pentoxifylline: A Drug with Antiviral and Anti-Inflammatory Effects to Be Considered in the Treatment of Coronavirus Disease 2019",
"author": "Ghasemnejad-Berenji",
"doi-asserted-by": "crossref",
"first-page": "98",
"issue": "1",
"journal-title": "Medical Principles and Practice.",
"key": "10.1016/j.intimp.2021.108227_b0040",
"volume": "30",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2018.01048",
"article-title": "Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases",
"author": "Li",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "Front. Pharmacol.",
"key": "10.1016/j.intimp.2021.108227_b0045",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1016/j.mehy.2020.109988",
"article-title": "Repositioning of pentoxifylline as an immunomodulator and regulator of the renin-angiotensin system in the treatment of COVID-19",
"author": "Maldonado",
"doi-asserted-by": "crossref",
"first-page": "109988",
"journal-title": "Med. Hypotheses",
"key": "10.1016/j.intimp.2021.108227_b0050",
"volume": "144",
"year": "2020"
},
{
"DOI": "10.1096/fj.13-228122",
"article-title": "Protective effects of pentoxifylline in pulmonary inflammation are adenosine receptor A2A dependent",
"author": "Konrad",
"doi-asserted-by": "crossref",
"first-page": "3524",
"issue": "9",
"journal-title": "FASEB J.",
"key": "10.1016/j.intimp.2021.108227_b0055",
"volume": "27",
"year": "2013"
},
{
"article-title": "Pentoxifylline in vivo down-regulates the release of IL-1 beta, IL-6, IL-8 and tumour necrosis factor-alpha by human peripheral blood mononuclear cells",
"author": "Neuner",
"first-page": "262",
"issue": "2",
"journal-title": "Immunology",
"key": "10.1016/j.intimp.2021.108227_b0060",
"volume": "83",
"year": "1994"
},
{
"DOI": "10.2165/11532600-000000000-00000",
"article-title": "Pentoxifylline in preterm neonates",
"author": "Harris",
"doi-asserted-by": "crossref",
"first-page": "301",
"issue": "5",
"journal-title": "Pediatric Drugs.",
"key": "10.1016/j.intimp.2021.108227_b0065",
"volume": "12",
"year": "2010"
},
{
"DOI": "10.1200/JCO.2004.09.101",
"article-title": "Pentoxifylline in the treatment of radiation-induced fibrosis",
"author": "Okunieff",
"doi-asserted-by": "crossref",
"first-page": "2207",
"issue": "11",
"journal-title": "J. Clin. Oncol.",
"key": "10.1016/j.intimp.2021.108227_b0070",
"volume": "22",
"year": "2004"
},
{
"article-title": "Pentoxifylline for the prevention of bronchopulmonary dysplasia in preterm infants",
"author": "Schulzke",
"journal-title": "Cochrane Database of Systematic Reviews.",
"key": "10.1016/j.intimp.2021.108227_b0075",
"volume": "11",
"year": "2014"
},
{
"article-title": "New properties of trental as an inhibitor of viral activity with a wide range of activity",
"author": "Amvros' eva",
"first-page": "230",
"issue": "5",
"journal-title": "Voprosy virusologii.",
"key": "10.1016/j.intimp.2021.108227_b0080",
"volume": "38",
"year": "1993"
},
{
"DOI": "10.1182/blood.V77.8.1653.bloodjournal7781653",
"doi-asserted-by": "crossref",
"key": "10.1016/j.intimp.2021.108227_b0085",
"unstructured": "F. Fazely, B.J. Dezube, J. Allen-Ryan, A.B. Pardee, R.M. Ruprecht, Pentoxifylline (Trental) decreases the replication of the human immunodeficiency virus type 1 in human peripheral blood mononuclear cells and in cultured T cells [see comments]. 1991."
},
{
"DOI": "10.3748/wjg.15.1613",
"article-title": "Pentoxifylline versus prednisolone for severe alcoholic hepatitis: a randomized controlled trial",
"author": "De",
"doi-asserted-by": "crossref",
"first-page": "1613",
"issue": "13",
"journal-title": "World journal of gastroenterology: WJG.",
"key": "10.1016/j.intimp.2021.108227_b0090",
"volume": "15",
"year": "2009"
},
{
"DOI": "10.1097/QAD.0b013e3283396024",
"article-title": "Anti-inflammatory treatment with pentoxifylline improves HIV-related endothelial dysfunction: a pilot study",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1377",
"issue": "9",
"journal-title": "AIDS (London, England)",
"key": "10.1016/j.intimp.2021.108227_b0095",
"volume": "24",
"year": "2010"
},
{
"DOI": "10.1016/S1665-2681(19)31363-8",
"article-title": "Addition of pentoxifylline to pegylated interferon-alpha-2a and ribavirin improves sustained virological response to chronic hepatitis C virus: a randomized clinical trial",
"author": "Jiménez-Luévano",
"doi-asserted-by": "crossref",
"first-page": "248",
"issue": "2",
"journal-title": "Annals of hepatology.",
"key": "10.1016/j.intimp.2021.108227_b0100",
"volume": "12",
"year": "2013"
},
{
"article-title": "Pentoxifylline and severe acute respiratory syndrome (SARS): a drug to be considered",
"author": "Martín",
"first-page": "SR29",
"issue": "6",
"journal-title": "Med. Sci. Monit.",
"key": "10.1016/j.intimp.2021.108227_b0105",
"volume": "9",
"year": "2003"
},
{
"author": "Organization",
"key": "10.1016/j.intimp.2021.108227_b0110",
"series-title": "Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020",
"year": "2020"
},
{
"author": "Organization",
"key": "10.1016/j.intimp.2021.108227_b0115",
"series-title": "Clinical management of COVID-19: interim guidance, 27 May 2020",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"issue": "19",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.intimp.2021.108227_b0120",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1111/j.1365-2710.2009.01077.x",
"article-title": "Effects of pentoxifylline on coagulation profile and disseminated intravascular coagulation incidence in Egyptian septic neonates",
"author": "Adel",
"doi-asserted-by": "crossref",
"first-page": "257",
"issue": "3",
"journal-title": "J. Clin. Pharm. Ther.",
"key": "10.1016/j.intimp.2021.108227_b0125",
"volume": "35",
"year": "2010"
},
{
"DOI": "10.1177/030089169608200117",
"article-title": "Characterization of cancer-related disseminated intravascular coagulation in relation to tumor necrosis factor-alpha blood concentrations: Possible therapeutic role of pentoxifylline",
"author": "Lissoni",
"doi-asserted-by": "crossref",
"first-page": "78",
"issue": "1",
"journal-title": "Tumori Journal.",
"key": "10.1016/j.intimp.2021.108227_b0130",
"volume": "82",
"year": "1996"
},
{
"DOI": "10.1016/j.intimp.2020.107209",
"article-title": "Pentoxifylline decreases serum LDH levels and increases lymphocyte count in COVID-19 patients: results from an external pilot study",
"author": "Maldonado",
"doi-asserted-by": "crossref",
"first-page": "107209",
"journal-title": "International Immunopharmacology.",
"key": "10.1016/j.intimp.2021.108227_b0135",
"volume": "90",
"year": "2021"
},
{
"DOI": "10.1111/crj.13363",
"article-title": "Pentoxifylline or theophylline use in hospitalized COVID-19 patients requiring oxygen support",
"author": "Wall",
"doi-asserted-by": "crossref",
"first-page": "843",
"issue": "7",
"journal-title": "The Clinical Respiratory Journal.",
"key": "10.1016/j.intimp.2021.108227_b0140",
"volume": "15",
"year": "2021"
},
{
"DOI": "10.1016/j.csbj.2021.02.009",
"article-title": "Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19",
"author": "Chavarría",
"doi-asserted-by": "crossref",
"first-page": "1379",
"journal-title": "Comput. Struct. Biotechnol. J.",
"key": "10.1016/j.intimp.2021.108227_b0145",
"volume": "19",
"year": "2021"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S1567576921008638"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "PB",
"subject": [],
"subtitle": [],
"title": "Pentoxifylline effects on hospitalized patients with COVID19: A randomized, double-blind clinical trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "101"
}