Evaluation of the efficacy of oral nano‐silymarin formulation in hospitalized patients with COVID‐19: A double‐blind placebo‐controlled clinical trial
et al., Phytotherapy Research, doi:10.1002/ptr.7537, IRCT20201024049130N1, Jul 2022
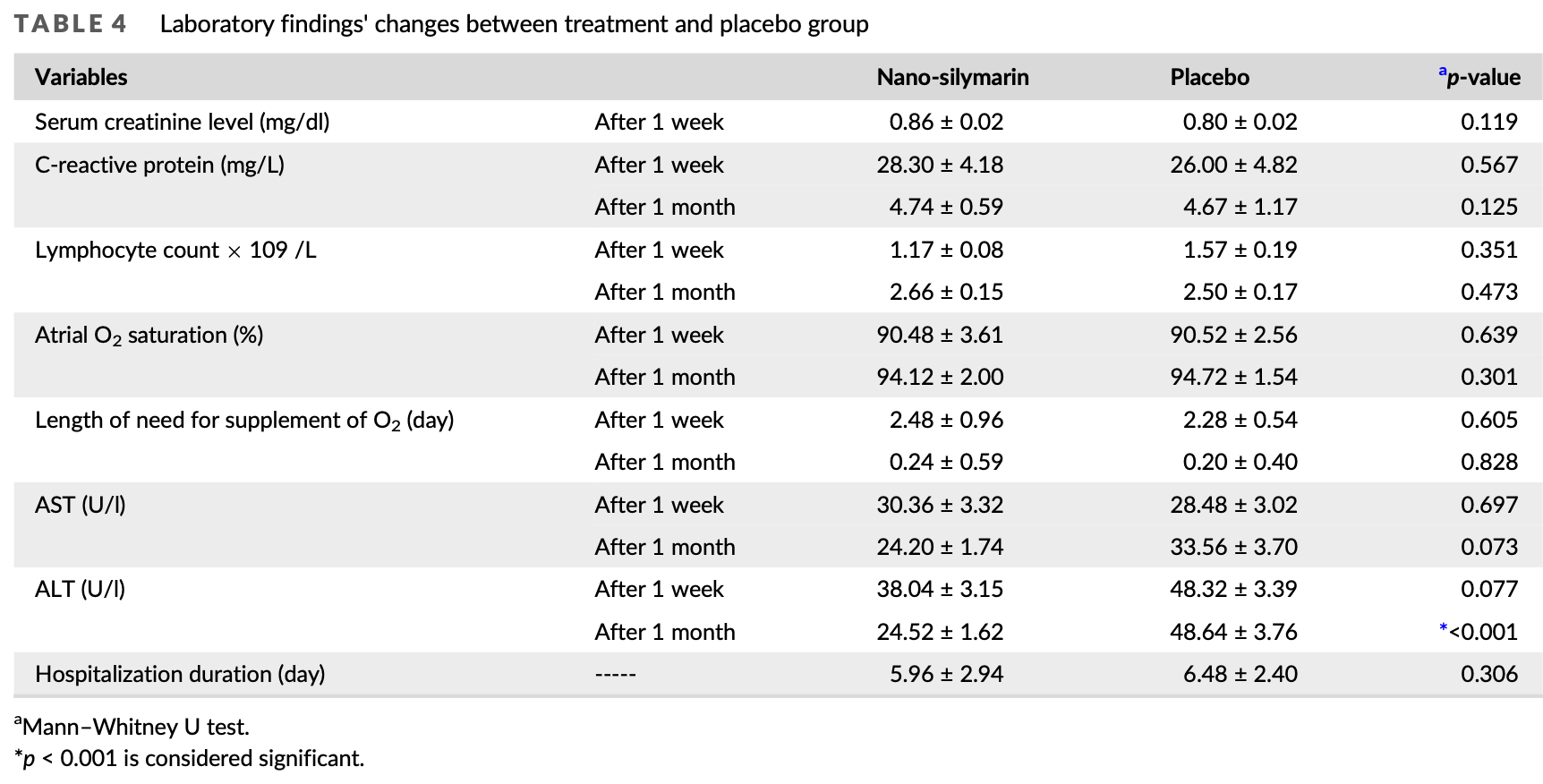
RCT 50 hospitalized COVID-19 patients showing no significant difference in symptom resolution time or hospitalization duration with nano-silymarin treatment.
|
oxygen time, 20.0% higher, relative time 1.20, p = 0.78, treatment mean 0.24 (±0.59) n=25, control mean 0.2 (±0.4) n=25, relative length of supplemental O2 required, day 30.
|
|
oxygen time, 8.8% higher, relative time 1.09, p = 0.37, treatment mean 2.48 (±0.96) n=25, control mean 2.28 (±0.54) n=25, relative length of supplemental O2 required, day 7.
|
|
hospitalization time, 8.0% lower, relative time 0.92, p = 0.50, treatment mean 5.96 (±2.94) n=25, control mean 6.48 (±2.4) n=25.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Aryan et al., 20 Jul 2022, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, mean age 49.0, 9 authors, study period March 2021 - September 2021, trial IRCT20201024049130N1.
Contact: reza.mosaed@ajaums.ac.ir.
Evaluation of the efficacy of oral nano‐silymarin formulation in hospitalized patients with COVID‐19: A double‐blind placebo‐controlled clinical trial
Phytotherapy Research, doi:10.1002/ptr.7537
Considering the outbreak pandemic of Coronavirus Disease 2019 (COVID-19), the lack of effective therapeutic strategies for the management of this viral disease, and the increasing evidence on the antiviral potential of silymarin, this study aimed to investigate the effectiveness of silymarin nanomicelles on the symptom's resolution time, laboratory parameters, and liver enzymes in patients with COVID-19. The participants were assigned to the nano-silymarin (n = 25) (receiving SinaLive soft gel, containing 70 mg silymarin as nanomicelles) or placebo groups (n = 25) three times daily for two weeks. Patients' symptoms and laboratory findings were assessed at baseline and during the follow-up period (one week and one month after the beginning of the treatment). No significant differences were observed between the two groups in terms of symptoms resolution time, laboratory parameters, and hospitalization duration (p > 0.05). However, the alanine aminotransferase level decreased significantly in the treatment group, compared to the placebo group (p < 0.001). Concomitant use of dexamethasone and remdesivir with silymarin might make the effects of silymarin on the improvement of patients' condition unclear. Further clinical trials are recommended with diverse dosages and larger sample sizes.
CONFLICT OF INTEREST Dr. Mahmoud Reza Jaafari, one of the manuscript authors, is the founder of Exir Nano Sina Company which produced the studied medication. Other authors have nothing to declare.
References
Abbasirad, Shaygannejad, Hosseininasab, Mirmosayyeb, Mahaki et al., Significant immunomodulatory and hepatoprotective impacts of Silymarin in MS patients: A double-blind placebo-controlled clinical trial, International Immunopharmacology
Badraoui, Alrashedi, El-May, Bardakci, Acute respiratory distress syndrome: A life threatening associated complication of SARS-CoV-2 infection inducing COVID-19, Journal of Biomolecular Structure and Dynamics
Bosch-Barrera, Martin-Castillo, Bux O, Brunet, Encinar et al., Silibinin and SARS-CoV-2: Dual targeting of host cytokine storm and virus replication machinery for clinical management of COVID-19 patients, Journal of Clinical Medicine
Brooks, Webster, Smith, Woodland, Wessely et al., The psychological impact of quarantine and how to reduce it: Rapid review of the evidence, The Lancet
Chen, Feng, Xu, Huang, Sun et al., Patterns of deterioration in moderate patients with COVID-19: A multi-center, retrospective cohort study, Frontiers in Medicine
Di Costanzo, Angelico, Formulation strategies for enhancing the bioavailability of silymarin: The state of the art, Molecules
Gorla, Rao, Kulandaivelu, Alavala, Panda, Lead finding from selected flavonoids with antiviral (SARS-CoV-2) potentials against COVID-19: An in-silico evaluation, Combinatorial Chemistry & High Throughput Screening
Guo, Cao, Hong, Tan, Chen et al., The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak-an update on the status, Military Medical Research
He, Hou, Lu, Zhu, Feng, Preparation, pharmacokinetics and body distribution of silymarin-loaded solid lipid nanoparticles after oral administration, Journal of Biomedical Nanotechnology
Hosseini, Rezaei, Moghaddam, Elyasi, Karimi, Evaluation of oral nano-silymarin formulation efficacy on prevention of radiotherapy induced mucositis: A randomized, doubleblinded, placebo-controlled clinical trial, Pharma Nutrition
Hussain, Jassim, Numan, Al-Khalifa, Abdullah, Anti-inflammatory activity of silymarin in patients with knee osteoarthritis, Saudi Medical Journal
Javed, Kohli, Ali, Reassessing bioavailability of silymarin, Alternative Medicine Review: A Journal of Clinical Therapeutic
Li, Huang, Wang, Wang, Liang et al., COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis, Journal of Medical Virology
Lovelace, Wagoner, Macdonald, Bammler, Bruckner et al., Silymarin suppresses cellular inflammation by inducing reparative stress signaling, Journal of Natural Products
Mahi-Birjand, Karimzadeh, Zarban, Abdollahpour-Alitappeh, Saadatjoo et al., Protective effects of silymarin on gentamicin-induced nephrotoxicity in infectious patients: A randomized double blinded placebo-controlled clinical trial, Pharmaceutical Sciences
Mcclure, Lovelace, Elahi, Maurice, Wagoner et al., Silibinin inhibits HIV-1 infection by reducing cellular activation and proliferation, PLoS ONE
Meng, Ling, Zhang, Zhang, Dong et al., Potential for jaktinib hydrochloride to treat cytokine storms in patients with COVID-19, Bioscience trends
Mirzaei, Sabetian, Masjedi, Heidari, Mirjalili et al., The effect of silymarin on liver enzymes and antioxidant status in trauma patients in the intensive care unit: A randomized double blinded placebo-controlled clinical trial, Clinical and Experimental Hepatology
Palit, Mukhopadhyay, Chattopadhyay, Phytopharmacological perspective of Silymarin: A potential prophylactic or therapeutic agent for COVID-19, based on its promising immunomodulatory, anti-coagulant and anti-viral property, Phytotherapy Research: PTR
Pan, Ye, Sun, Gui, Liang et al., Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19), Radiology
Parveen, Baboota, Ali, Ahuja, Vasudev et al., Oil based nanocarrier for improved oral delivery of silymarin: In vitro and in vivo studies, International Journal of Pharmaceutics
Piazzini, D'ambrosio, Luceri, Cinci, Landucci et al., Formulation of Nanomicelles to improve the solubility and the Oral absorption of Silymarin, Molecules, doi:10.3390/molecules24091688
Rahmanzade, Rahmanzadeh, Hashemian, Tabarsi, Iran's approach to COVID-19: Evolving treatment protocols and ongoing clinical trials, Frontiers Public Health
Robinson, Richards, Tanner, Feldmann, Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment, The Lancet Rheumatology
Roozbeh, Shahriyari, Akmali, Vessal, Pakfetrat et al., Comparative effects of silymarin and vitamin E supplementation on oxidative stress markers, and hemoglobin levels among patients on hemodialysis, Renal Failure
Sardanelli, Isgrò, Palese, SARS-CoV-2 Main protease active site ligands in the human metabolome, Molecules
Sarzi-Puttini, Giorgi, Sirotti, Marotto, Ardizzone et al., COVID-19, cytokines and immunosuppression: What can we learn from severe acute respiratory syndrome?, Clinical and Experimental Rheumatology
Song, Choi, Silymarin efficacy against influenza a virus replication, Phytomedicine: International Journal of Phytotherapy and Phytopharmacology
Tanamly, Tadros, Labeeb, Makld, Shehata et al., Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: Study description and 12-month results, Digestive and Liver Disease
Tian, Li, Wang, Therapeutic effects of silibinin on LPS-induced acute lung injury by inhibiting NLRP3 and NF-κB signaling pathways, Microbial Pathogenesis
Umetsu, Inoue, Kogure, Kakazu, Ninomiya et al., Inhibitory effect of silibinin on hepatitis B virus entry, Biochemistry and Biophysics reports
Wagoner, Negash, Kane, Martinez, Nahmias et al., Multiple effects of silymarin on the hepatitis C virus lifecycle, Hepatology
Whitehead, Julious, Cooper, Campbell, Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable, Statistical Methods in Medical Research
Zhang, Wang, Cao, Wang, Wu, Silybin attenuates LPS-induced lung injury in mice by inhibiting NF-κB signaling and NLRP3 activation, International Journal of Molecular Medicine
Zhang, Zhao, Zhang, Wang, Li et al., The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The perspectives of clinical immunologists from China, Clinical Immunology and Immunopathology
DOI record:
{
"DOI": "10.1002/ptr.7537",
"ISSN": [
"0951-418X",
"1099-1573"
],
"URL": "http://dx.doi.org/10.1002/ptr.7537",
"abstract": "<jats:title>Abstract</jats:title><jats:p>Considering the outbreak pandemic of Coronavirus Disease 2019 (COVID‐19), the lack of effective therapeutic strategies for the management of this viral disease, and the increasing evidence on the antiviral potential of silymarin, this study aimed to investigate the effectiveness of silymarin nanomicelles on the symptom's resolution time, laboratory parameters, and liver enzymes in patients with COVID‐19. The participants were assigned to the nano‐silymarin (<jats:italic>n</jats:italic> = 25) (receiving SinaLive soft gel, containing 70 mg silymarin as nanomicelles) or placebo groups (<jats:italic>n</jats:italic> = 25) three times daily for two weeks. Patients' symptoms and laboratory findings were assessed at baseline and during the follow‐up period (one week and one month after the beginning of the treatment). No significant differences were observed between the two groups in terms of symptoms resolution time, laboratory parameters, and hospitalization duration (<jats:italic>p</jats:italic> > 0.05). However, the alanine aminotransferase level decreased significantly in the treatment group, compared to the placebo group (<jats:italic>p</jats:italic> < 0.001). Concomitant use of dexamethasone and remdesivir with silymarin might make the effects of silymarin on the improvement of patients' condition unclear. Further clinical trials are recommended with diverse dosages and larger sample sizes.</jats:p>",
"alternative-id": [
"10.1002/ptr.7537"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2021-12-16"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2022-06-12"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2022-07-20"
}
],
"author": [
{
"affiliation": [
{
"name": "Department of Anesthesiology and Intensive Care Aja University of Medical Sciences Tehran Iran"
}
],
"family": "Aryan",
"given": "Hossein",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Infectious Disease Research Center AJA University of Medical Sciences Tehran Iran"
}
],
"family": "Farahani",
"given": "Ramin Hamidi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Toxicology Research Center Aja University of Medical Sciences Tehran Iran"
},
{
"name": "Department of Pharmacology, School of Medicine Aja University of Medical Sciences Tehran Iran"
}
],
"family": "Chamanara",
"given": "Mohsen",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9857-1175",
"affiliation": [
{
"name": "Department of Clinical Pharmacy School of Pharmacy Mashhad University of Medical Sciences Mashhad Iran"
}
],
"authenticated-orcid": false,
"family": "Elyasi",
"given": "Sepideh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nanotechnology Research Center Pharmaceutical Technology Institute, Mashhad University of Medical Sciences Mashhad Iran"
},
{
"name": "Department of Pharmaceutical Nanotechnology School of Pharmacy, Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Jaafari",
"given": "Mahmoud Reza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pharmaceutical Nanotechnology School of Pharmacy, Mashhad University of Medical Sciences Mashhad Iran"
}
],
"family": "Haddad",
"given": "Mahboubeh",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases and Tropical medicine Faculty of medicine, Mashhad University of medical sciences Mashhad Iran"
}
],
"family": "Sani",
"given": "Ashraf Tavanaee",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Medicine Mashhad University of Medical Sciences Mashhad Mashhad Iran"
}
],
"family": "Ardalan",
"given": "Mohamed Afshar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7819-9668",
"affiliation": [
{
"name": "Internal Medicine Department School of Medicine, AJA University of Medical Sciences Tehran Iran"
}
],
"authenticated-orcid": false,
"family": "Mosaed",
"given": "Reza",
"sequence": "additional"
}
],
"container-title": "Phytotherapy Research",
"container-title-short": "Phytotherapy Research",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2022,
7,
21
]
],
"date-time": "2022-07-21T04:28:44Z",
"timestamp": 1658377724000
},
"deposited": {
"date-parts": [
[
2023,
8,
21
]
],
"date-time": "2023-08-21T20:40:12Z",
"timestamp": 1692650412000
},
"indexed": {
"date-parts": [
[
2025,
4,
12
]
],
"date-time": "2025-04-12T11:26:38Z",
"timestamp": 1744457198466
},
"is-referenced-by-count": 9,
"issue": "10",
"issued": {
"date-parts": [
[
2022,
7,
20
]
]
},
"journal-issue": {
"issue": "10",
"published-print": {
"date-parts": [
[
2022,
10
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://onlinelibrary.wiley.com/termsAndConditions#vor",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
7,
20
]
],
"date-time": "2022-07-20T00:00:00Z",
"timestamp": 1658275200000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7537",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/full-xml/10.1002/ptr.7537",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/ptr.7537",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "3924-3931",
"prefix": "10.1002",
"published": {
"date-parts": [
[
2022,
7,
20
]
]
},
"published-online": {
"date-parts": [
[
2022,
7,
20
]
]
},
"published-print": {
"date-parts": [
[
2022,
10
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.intimp.2021.107715",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_2_1"
},
{
"DOI": "10.1080/07391102.2020.1803139",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_3_1"
},
{
"DOI": "10.3390/jcm9061770",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_4_1"
},
{
"DOI": "10.1016/S0140-6736(20)30460-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_5_1"
},
{
"article-title": "Patterns of deterioration in moderate patients with COVID‐19: A multi‐center, retrospective cohort study",
"author": "Chen S.‐L.",
"first-page": "839",
"journal-title": "Frontiers in Medicine",
"key": "e_1_2_9_6_1",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3390/molecules24112155",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_7_1"
},
{
"DOI": "10.2174/18755402MTA5vMjMn5",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_8_1"
},
{
"DOI": "10.1186/s40779-020-00240-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_9_1"
},
{
"DOI": "10.1166/jbn.2007.024",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_10_1"
},
{
"DOI": "10.1016/j.phanu.2021.100253",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_11_1"
},
{
"article-title": "Anti‐inflammatory activity of silymarin in patients with knee osteoarthritis",
"author": "Hussain S. A.",
"first-page": "98",
"issue": "1",
"journal-title": "Saudi Medical Journal",
"key": "e_1_2_9_12_1",
"volume": "30",
"year": "2009"
},
{
"article-title": "Reassessing bioavailability of silymarin",
"author": "Javed S.",
"first-page": "239",
"issue": "3",
"journal-title": "Alternative Medicine Review: A Journal of Clinical Therapeutic",
"key": "e_1_2_9_13_1",
"volume": "16",
"year": "2011"
},
{
"DOI": "10.1002/jmv.25757",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_14_1"
},
{
"DOI": "10.1021/acs.jnatprod.5b00288",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_15_1"
},
{
"DOI": "10.34172/PS.2020.33",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_16_1"
},
{
"DOI": "10.1371/journal.pone.0041832",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_17_1"
},
{
"DOI": "10.5582/bst.2020.03106",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_18_1"
},
{
"DOI": "10.5114/ceh.2021.107067",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_19_1"
},
{
"DOI": "10.1002/ptr.7084",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_20_1"
},
{
"DOI": "10.1148/radiol.2020200370",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_21_1"
},
{
"DOI": "10.1016/j.ijpharm.2011.04.041",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_22_1"
},
{
"DOI": "10.3390/molecules24091688",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_23_1"
},
{
"article-title": "Iran's approach to COVID‐19: Evolving treatment protocols and ongoing clinical trials. Frontiers",
"author": "Rahmanzade R.",
"journal-title": "Public Health",
"key": "e_1_2_9_24_1",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S2665-9913(20)30309-X",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_25_1"
},
{
"DOI": "10.3109/0886022X.2010.541579",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_26_1"
},
{
"DOI": "10.3390/molecules26051409",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_27_1"
},
{
"DOI": "10.55563/clinexprheumatol/xcdary",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_28_1"
},
{
"DOI": "10.1016/j.phymed.2011.01.026",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_29_1"
},
{
"DOI": "10.1016/j.dld.2004.06.015",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_30_1"
},
{
"DOI": "10.1016/j.micpath.2017.05.011",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_31_1"
},
{
"DOI": "10.1016/j.bbrep.2018.03.003",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_32_1"
},
{
"DOI": "10.1002/hep.23587",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_33_1"
},
{
"DOI": "10.1177/0962280215588241",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_34_1"
},
{
"DOI": "10.3892/ijmm.2017.2935",
"doi-asserted-by": "publisher",
"key": "e_1_2_9_35_1"
},
{
"article-title": "The use of anti‐inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID‐19): The perspectives of clinical immunologists from China",
"author": "Zhang W.",
"first-page": "108393",
"journal-title": "Clinical Immunology and Immunopathology",
"key": "e_1_2_9_36_1",
"volume": "214",
"year": "2020"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/ptr.7537"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Evaluation of the efficacy of oral nano‐silymarin formulation in hospitalized patients with COVID‐19: A double‐blind placebo‐controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "36"
}