Sep 16 2024 |
et al., AMB Express, doi:10.1186/s13568-024-01739-8 | Establishment of in-house assay for screening of anti-SARS-CoV-2 protein inhibitors |
| In vitro study showing that curcumin, quercetin, gallic acid, and silymarin inhibit SARS-CoV-2 spike protein binding to the ACE2 receptor. Authors developed a novel immunofluorescent assay to screen potential inhibitors of the spike-ACE2 .. | ||
Jul 20 2022 |
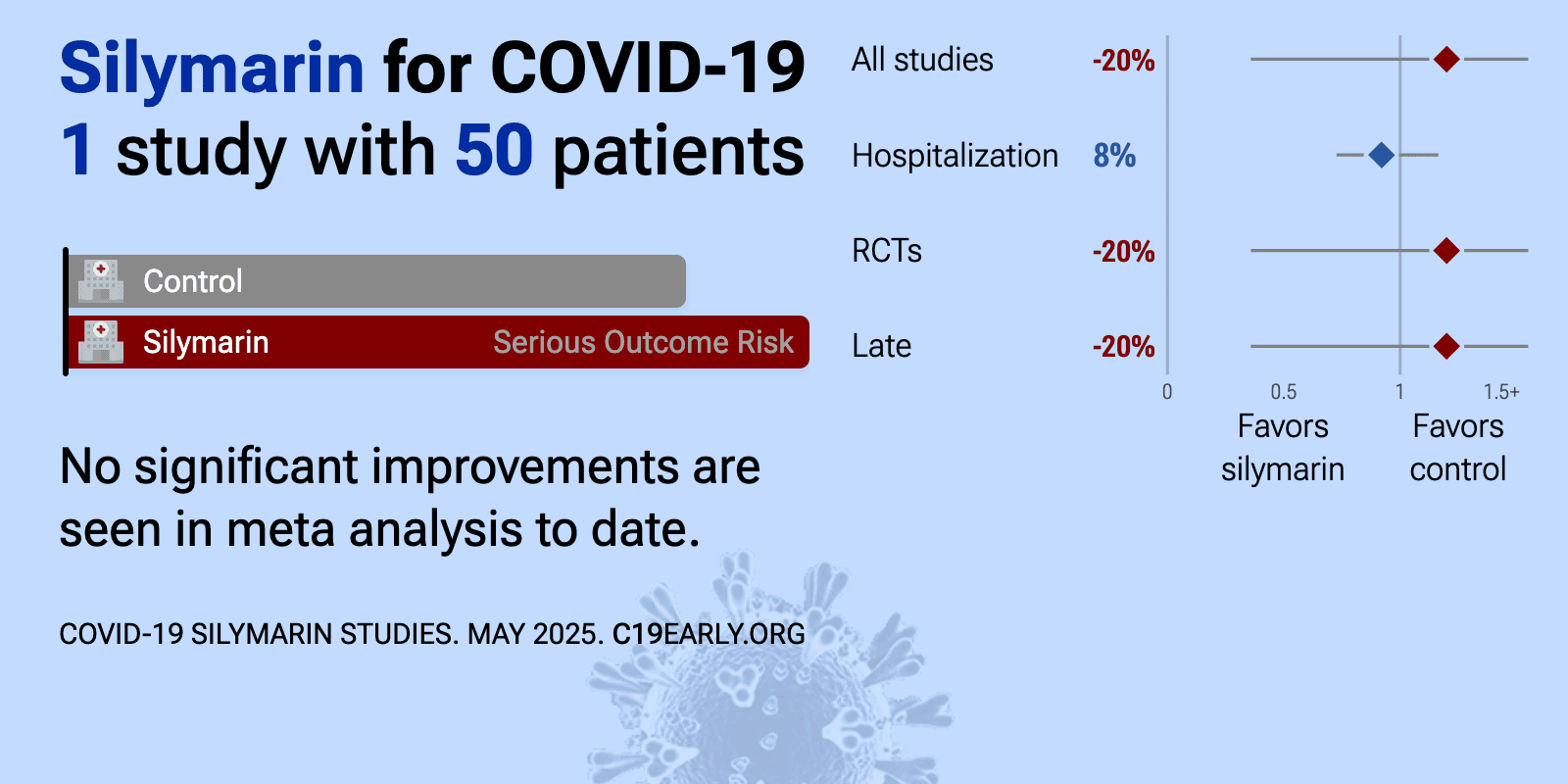
et al., Phytotherapy Research, doi:10.1002/ptr.7537 | Evaluation of the efficacy of oral nano‐silymarin formulation in hospitalized patients with COVID‐19: A double‐blind placebo‐controlled clinical trial |
| 20% higher need for oxygen therapy (p=0.78) and 8% shorter hospitalization (p=0.5). RCT 50 hospitalized COVID-19 patients showing no significant difference in symptom resolution time or hospitalization duration with nano-silymarin treatment. | ||