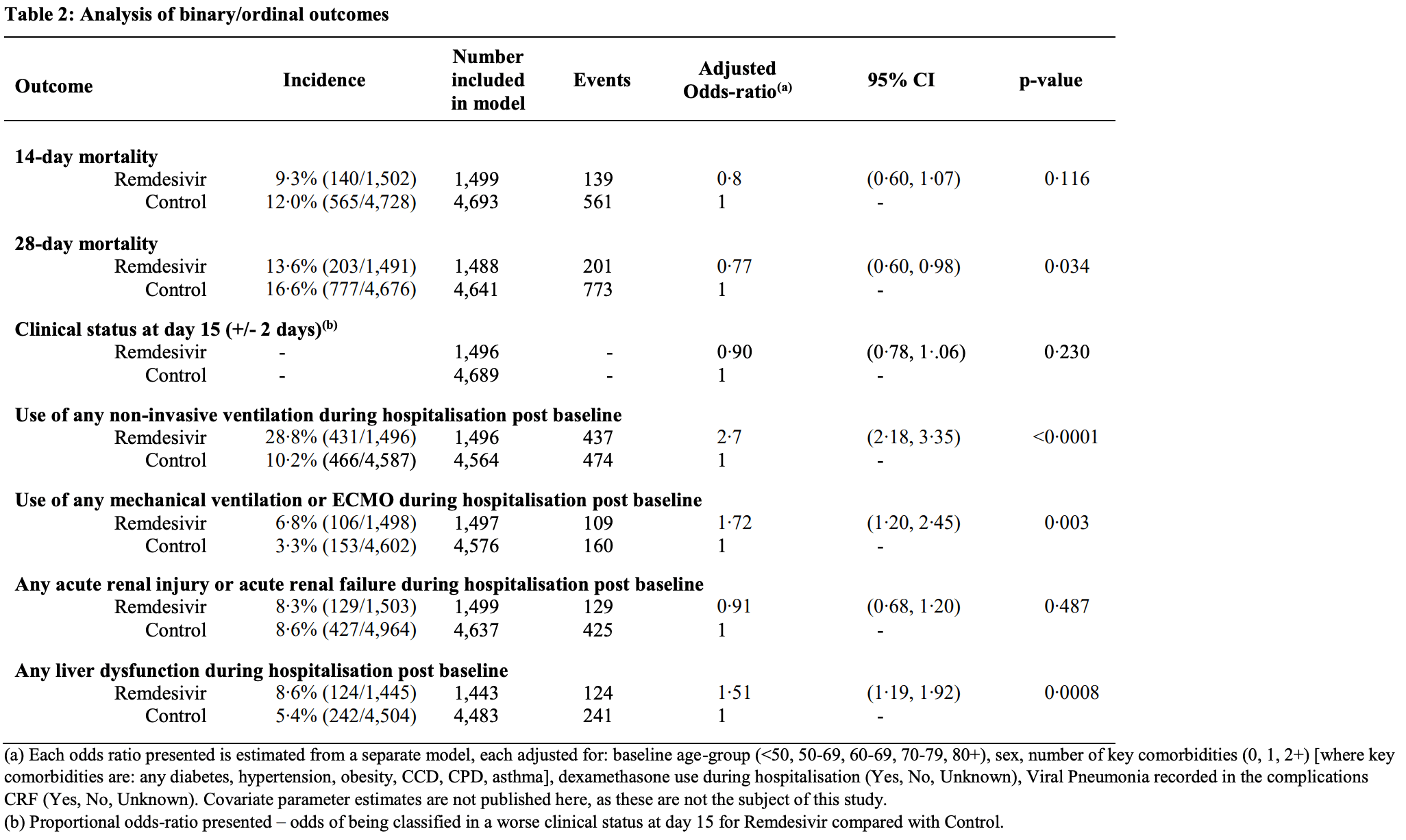
Evaluation of the effectiveness of remdesivir in treating severe COVID-19 using data from the ISARIC WHO Clinical Characterisation Protocol UK: a prospective, national cohort study
et al., medRxiv, doi:10.1101/2021.06.18.21259072, Jun 2021
Prospective PSM analysis of remdesivir use in the UK showing statistically significantly lower mortality at 28 days. For unspecified reasons, the study prioritized short-term outcomes. Mortality at 14 days was also lower but not statistically significant. Confounding by indication is likely and may only be partially addressed by the variables included in the PSM.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
Remdesivir efficacy disappears with longer
followup. Mixed-effects meta-regression of efficacy as a function of
followup duration across all remdesivir studies shows decreasing efficacy with
longer followup15. This may reflect
antiviral efficacy being offset by serious adverse effects of treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the United Kingdom, is very poor with very low average efficacy for approved treatments16.
The United Kingdom focused on expensive high-profit treatments, approving only one low-cost early treatment, which required a prescription and had limited adoption. The high-cost prescription treatment strategy reduces the probability of early treatment due to access and cost barriers, and eliminates complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 19.9% lower, RR 0.80, p = 0.03, treatment 203 of 1,491 (13.6%), control 777 of 4,676 (16.6%), NNT 33, odds ratio converted to relative risk, PSM, day 28.
|
|
risk of death, 18.0% lower, RR 0.82, p = 0.12, treatment 140 of 1,502 (9.3%), control 565 of 4,728 (12.0%), NNT 38, odds ratio converted to relative risk, PSM, day 14.
|
|
risk of mechanical ventilation, 68.0% higher, RR 1.68, p = 0.003, treatment 106 of 1,498 (7.1%), control 153 of 4,602 (3.3%), odds ratio converted to relative risk, PSM, day 28.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
13.
Mohammed et al., Bradycardia associated with remdesivir treatment in coronavirus disease 2019 patients: A propensity score-matched analysis, Medicine, doi:10.1097/MD.0000000000044501.
Arch et al., 21 Jun 2021, prospective, propensity score matching, United Kingdom, preprint, 10 authors, average treatment delay 6.0 days.
Abstract: medRxiv preprint doi: https://doi.org/10.1101/2021.06.18.21259072; this version posted June 21, 2021. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
Evaluation of the effectiveness of remdesivir in treating severe COVID-19
using data from the ISARIC WHO Clinical Characterisation Protocol UK:
a prospective, national cohort study.
Short Running title: ISARIC4C Remdesivir effectiveness study
Authors
Arch BN1, Kovacs D2*, Scott JT3*, Jones AP1, Harrison EM4, Rosala-Hallas A1, Gamble CG1, Openshaw
PJM 5, Baillie JK6, Semple MG7,8 on behalf of ISARIC4C Investigators
*Joint 2nd authors
Affiliations
1. Liverpool Clinical Trials Centre, Clinical Directorate, Faculty of Health and Life Sciences,
University of Liverpool, UK.
2. Institute of Biodiversity, Animal health and Comparative Medicine, University of Glasgow,
Glasgow, UK
3. MRC-University of Glasgow Centre for Virus Research, Glasgow, UK
4. Centre for Medical Informatics, The Usher Institute, University of Edinburgh, Edinburgh, UK
5. National Heart and Lung Institute, Imperial College London, London, UK
6. Roslin Institute, University of Edinburgh, Edinburgh, UK
7. Health Protection Research Unit in Emerging and Zoonotic Infections, Institute of Infection,
Veterinary and Ecological Sciences, Faculty of Health and Life Sciences, University of
Liverpool, Liverpool, UK.
8. Department of Respiratory Medicine, Alder Hey Children’s Hospital, Liverpool, UK.
Corresponding author
Barbara Arch. Liverpool Clinical Trials Centre, University of Liverpool, Institute in the Park, Alder
Hey Childrens Hospital, Eaton Road, Liverpool, L12 2AP.
Email: barbara.arch@liverpool.ac.uk, Tel: +44(0)151 795 8751
Word Count
Abstract 267/300
Manuscript (excluding Research in Context Panel) 3,579/3,500
NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice.
Page 1 of 22
medRxiv preprint doi: https://doi.org/10.1101/2021.06.18.21259072; this version posted June 21, 2021. The copyright holder for this preprint
(which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity.
It is made available under a CC-BY-NC-ND 4.0 International license .
Abstract
Background
Remdesivir was given UK early-access approval for use in COVID-19 in people aged 12 years and
older on 26th May 2020 on the basis of unmet clinical need. Evidence on the side effects, complications
of therapy and effectiveness of this therapy is lacking or conflicting.
Methods
Adults with severe COVID-19 treated with remdesivir were compared with propensity-score matched
controls, identified from the ISARIC WHO Clinical Characterisation Protocol study of UK hospitalised
patients with COVID-19. Remdesivir patients were matched to controls according to baseline
underlying 14-day mortality risk. The effect of remdesivir on short-term outcomes was investigated
(primary outcome: 14-day mortality). Effect sizes were estimated and adjusted for potential
confounders using multivariable modelling.
Results
1,549 patients given remdesivir and 4,964 matched controls were identified satisfying inclusion and
exclusion criteria. The balance diagnostic threshold was achieved. Patients had symptoms for a median
of 6 days prior to..
DOI record:
{
"DOI": "10.1101/2021.06.18.21259072",
"URL": "http://dx.doi.org/10.1101/2021.06.18.21259072",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Remdesivir has been evaluated in clinical trial populations, but there is a sparsity of evidence evaluating effectiveness in general populations.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Adults eligible to be treated with remdesivir, requiring oxygen but not ventilated, were identified from UK patients hospitalised with COVID-19. Patients treated with remdesivir within 24h of hospitalisation were compared with propensity-score matched controls; estimates of effectiveness were calculated for short-term outcomes (14-day mortality, 28-day mortality, time-to-recovery among others) using multivariable modelling.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>9,278 out of 39,330 patients satisfied eligibility criteria. 1,549 patients were identified as ‘treated’ and matched with 4,964 controls. Patients were 62% male, mean (SD) age 63.1 (15.6) years, 80% ‘White’ ethnicity, and symptomatic for a median of 6 days prior to baseline. There was no statistically significant benefit of remdesivir at 14 days in terms of mortality or clinical status; there were signals of effectiveness in time-to-recovery after day 9, and a reduction in 28-day mortality.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>In a real-world setting, initiation of remdesivir within 24h of hospitalisation in conjunction with standard of care was not associated with a benefit at 14 days but supports clinical trial evidence of a potential reduction in 28-day mortality.</jats:p></jats:sec>",
"accepted": {
"date-parts": [
[
2022,
3,
9
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0001-6060-8091",
"affiliation": [],
"authenticated-orcid": false,
"family": "Arch",
"given": "B N",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-2187-743X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kovacs",
"given": "D",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8030-5223",
"affiliation": [],
"authenticated-orcid": false,
"family": "Scott",
"given": "J T",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5253-730X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Jones",
"given": "A P",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5018-3066",
"affiliation": [],
"authenticated-orcid": false,
"family": "Harrison",
"given": "E M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8012-9995",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rosala-Hallas",
"given": "A",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3021-1955",
"affiliation": [],
"authenticated-orcid": false,
"family": "Gamble",
"given": "C G",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7220-2555",
"affiliation": [],
"authenticated-orcid": false,
"family": "Openshaw",
"given": "P J M",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5258-793X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Baillie",
"given": "J K",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9700-0418",
"affiliation": [],
"authenticated-orcid": false,
"family": "Semple",
"given": "M G",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
6,
21
]
],
"date-time": "2021-06-21T13:50:14Z",
"timestamp": 1624283414000
},
"deposited": {
"date-parts": [
[
2022,
3,
11
]
],
"date-time": "2022-03-11T16:15:21Z",
"timestamp": 1647015321000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2024,
3,
19
]
],
"date-time": "2024-03-19T20:16:30Z",
"timestamp": 1710879390079
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 8,
"issued": {
"date-parts": [
[
2021,
6,
21
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.06.18.21259072",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
6,
21
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
6,
21
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference": [
{
"key": "2022031108150491000_2021.06.18.21259072v2.1",
"unstructured": "NICE. COVID 19 rapid evidence summary: Remdesivir for treating hospitalised patients with suspected or confirmed COVID-19. 2020."
},
{
"DOI": "10.1101/2020.04.15.043166",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.2",
"unstructured": "Williamson BN , Feldmann F , Schwarz B , et al. Clinical benefit of remdesivir in rhesus macaques infected with SARS-CoV-2. 2020."
},
{
"DOI": "10.1038/nature17180",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.3"
},
{
"DOI": "10.1038/s41467-020-20542-0",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.4"
},
{
"DOI": "10.1126/scitranslmed.aal3653",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.5"
},
{
"DOI": "10.1128/mBio.00221-18",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.6",
"unstructured": "Agostini ML , Andres EL , Sims AC , et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018; 9."
},
{
"DOI": "10.1101/2020.04.03.024257",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.7",
"unstructured": "Bojkova D , McGreig JE , McLaughlin K-M , et al. SARS-CoV-2 and SARS-CoV differ in their cell tropism and drug sensitivity profiles. bioRxiv 2020."
},
{
"DOI": "10.1101/2020.04.03.20052548",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.8",
"unstructured": "De Meyer S , Bojkova D , Cinati J , et al. Lack of Antiviral Activity of Darunavir against SARS-CoV-2. 2020."
},
{
"DOI": "10.1101/2020.05.12.090035",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.9",
"unstructured": "Ko M , Jeon S , Ryu W-S , Kim S. Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells: Nafamostat is the most potent antiviral drug candidate. 2020."
},
{
"DOI": "10.21203/rs.3.rs-23951/v1",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.10",
"unstructured": "Ellinger B , Bojkova D , Zaliani A , et al. Identification of inhibitors of SARS-CoV-2 in-vitro cellular toxicity in human (Caco-2) cells using a large scale drug repurposing collection. 2020."
},
{
"DOI": "10.1101/2020.06.19.161042",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.11",
"unstructured": "Dittmar M , Lee JS , Whig K , et al. Drug repurposing screens reveal FDA approved drugs active against SARS-Cov-2. 2020."
},
{
"DOI": "10.1126/scitranslmed.abb5883",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.12",
"unstructured": "Sheahan TP , Sims AC , Zhou S , et al. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med 2020; 12."
},
{
"DOI": "10.2139/ssrn.3588829",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.13",
"unstructured": "Pruijssers AJ , George AS , Schäfer A , et al. Remdesivir potently inhibits SARS-CoV-2 in human lung cells and chimeric SARS-CoV expressing the SARS-CoV-2 RNA polymerase in mice. 2020."
},
{
"DOI": "10.1111/cts.12840",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.14"
},
{
"DOI": "10.1016/s0140-6736(20)31022-9",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.15"
},
{
"DOI": "10.1001/jama.2020.16349",
"article-title": "Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial",
"doi-asserted-by": "crossref",
"first-page": "1048",
"journal-title": "JAMA",
"key": "2022031108150491000_2021.06.18.21259072v2.16",
"volume": "324",
"year": "2020"
},
{
"key": "2022031108150491000_2021.06.18.21259072v2.17",
"unstructured": "Beigel JH , Tomashek KM , Dodd LE , et al. Remdesivir for the Treatment of Covid-19 — Final Report. New England Journal of Medicine 2020."
},
{
"article-title": "Repurposed Antiviral Drugs for Covid-19 — Interim WHO Solidarity Trial Results",
"first-page": "497",
"journal-title": "New England Journal of Medicine",
"key": "2022031108150491000_2021.06.18.21259072v2.18",
"volume": "384",
"year": "2020"
},
{
"key": "2022031108150491000_2021.06.18.21259072v2.19",
"unstructured": "ISARIC WHO CCP-UK Study materials Available at: https://isaric4c.net/protocols/. Accessed 04/05/2021."
},
{
"key": "2022031108150491000_2021.06.18.21259072v2.20",
"unstructured": "Interim Clinical Commissioning Policy: Remdesivir for patients hospitalised with COVID-19 (adults and children 12 years and older) Version 2."
},
{
"key": "2022031108150491000_2021.06.18.21259072v2.21",
"unstructured": "Arch BN . Remdesivir Effectiveness Study Statistical Analysis Plan Available at: https://isaric4c.net/outputs/remdesivir/. Accessed 04/05/2021."
},
{
"DOI": "10.1136/bmj.m3339",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.22"
},
{
"DOI": "10.1214/09-STS313",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.23"
},
{
"DOI": "10.1016/j.jclinepi.2013.01.013",
"doi-asserted-by": "publisher",
"key": "2022031108150491000_2021.06.18.21259072v2.24"
},
{
"DOI": "10.18637/jss.v042.i08",
"doi-asserted-by": "crossref",
"key": "2022031108150491000_2021.06.18.21259072v2.25",
"unstructured": "Ho DE , Imai K , G K, Stuart EA . MatchIt: Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw 2011; 42(8)."
},
{
"DOI": "10.1056/NEJMoa2015301",
"article-title": "Remdesivir for 5 or 10 Days in Patients with Severe Covid-19",
"doi-asserted-by": "crossref",
"first-page": "1827",
"journal-title": "N Engl J Med",
"key": "2022031108150491000_2021.06.18.21259072v2.26",
"volume": "383",
"year": "2020"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "http://medrxiv.org/lookup/doi/10.1101/2021.06.18.21259072"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": "Evaluation of the effectiveness of remdesivir in severe COVID-19 using observational data from a prospective national cohort study",
"type": "posted-content"
}