Industry Alliance Platform Trial to Assess the Efficacy and Safety of Multiple Candidate Agents for the Treatment of COVID-19 in Hospitalized Patients
, NCT04590586, COMMUNITY, NCT04590586, Apr 2022
RCT 55 hospitalized patients showing no significant difference with lanadelumab treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
This may explain in part the very high mortality seen in this study.
Results may differ in countries with improved SOC.
Study covers apremilast and lanadelumab.
|
risk of death, 6.7% higher, RR 1.07, p = 1.00, treatment 8 of 25 (32.0%), control 9 of 30 (30.0%).
|
|
risk of no improvement, 10.0% lower, RR 0.90, p = 0.79, treatment 9 of 25 (36.0%), control 12 of 30 (40.0%), NNT 25.
|
|
risk of no hospital discharge, 23.6% lower, RR 0.76, p = 0.57, treatment 7 of 25 (28.0%), control 11 of 30 (36.7%), NNT 12.
|
|
risk of no recovery, 9.1% higher, RR 1.09, p = 1.00, treatment 10 of 25 (40.0%), control 11 of 30 (36.7%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Amgen et al., 7 Apr 2022, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04590586 (history) (COMMUNITY).
Contact: medinfo@amgen.com.
Abstract: Summary of clinical study results
Title of the study:
Industry Alliance Platform Trial to Assess the Efficacy and Safety of Multiple Candidate Agents
for the Treatment of COVID-19 in Hospitalized Patients
Short title of the study:
COVID-19 Multiple Agents and Modulators Unified Industry Members Trial (COMMUNITY)
What was the study about?
o learn whether 3 investigational medications worked and how safe they were. The
T
investigational medications were studied in participants hospitalized with a SARS-CoV-2
infection. SARS-CoV-2 is the virus that causes COVID-19.
Who sponsored the study?
mgen Inc. supported by its industry partners Takeda Development Center Americas, Inc and
A
UCB Biopharma SRL
What was tested?
here were 3 investigational medications tested: apremilast (marketed in some countries as
T
Otezla®), lanadelumab-IV, and zilucoplan (RA101495).
Thank you to all participants
We would like to thank everyone who took part in this study. Every participant in this study
helped the researchers learn more about the treatment of COVID-19.
The sponsor is committed to making research results available to the public. This summary
has been provided as part of that commitment and should not be used for any other purpose.
It should not be considered to make a claim for any product or to guide treatment decisions.
Some information in this summary involving apremilast may be different from the prescribing
information that doctors consider when they give patients this medicine that is approved for
unrelated conditions.
About this summary
This summary was completed in April 2022.
Where can I learn more about the study?
You can find more information on the websites listed at the end of this summary.
1
At a glance
Why was the research needed?
Researchers were looking for a better way to treat people hospitalized with COVID-19. Researchers
look at the results of many studies to understand if a medicine might work for a specific condition.
It takes a lot of people in many studies all around the world to advance medical science.
Who took part in the study?
8
515
Countries
Adults aged 18 years or over who were hospitalized with COVID-19 were
eligible to take part. A total of 515 people took part across 8 countries.
What study medicine did participants receive?
Apremilast Lanadelumab-IV Zilucoplan
There were 3 investigational medications studied:
apremilast, lanadelumab-IV, and zilucoplan.
Participants were assigned to 1 of the 3
investigational medications or a placebo.
A placebo looks like an investigational medication, but has no actual medicine in it.
Placebo is used to better see the effects of the investigational medications being tested.
Placebo
Any of the 3 investigational medications or the placebo may be referred to as the
“study medicine”.
All participants also received the standard treatment (standard of care) for COVID-19 that
was available locally.
What were the main results of the study?
The main question the researchers wanted to answer was:
• Did any of the 3 investigational medications affect the time it took for people in the
hospital to recover from COVID-19?
Researchers also wanted to see if, after about a month from starting in the study, any of the
3 investigational medications affected the number of people who:
• improved or were able to leave the hospital
• had improved, been released from the hospital, and who no longer needed extra oxygen
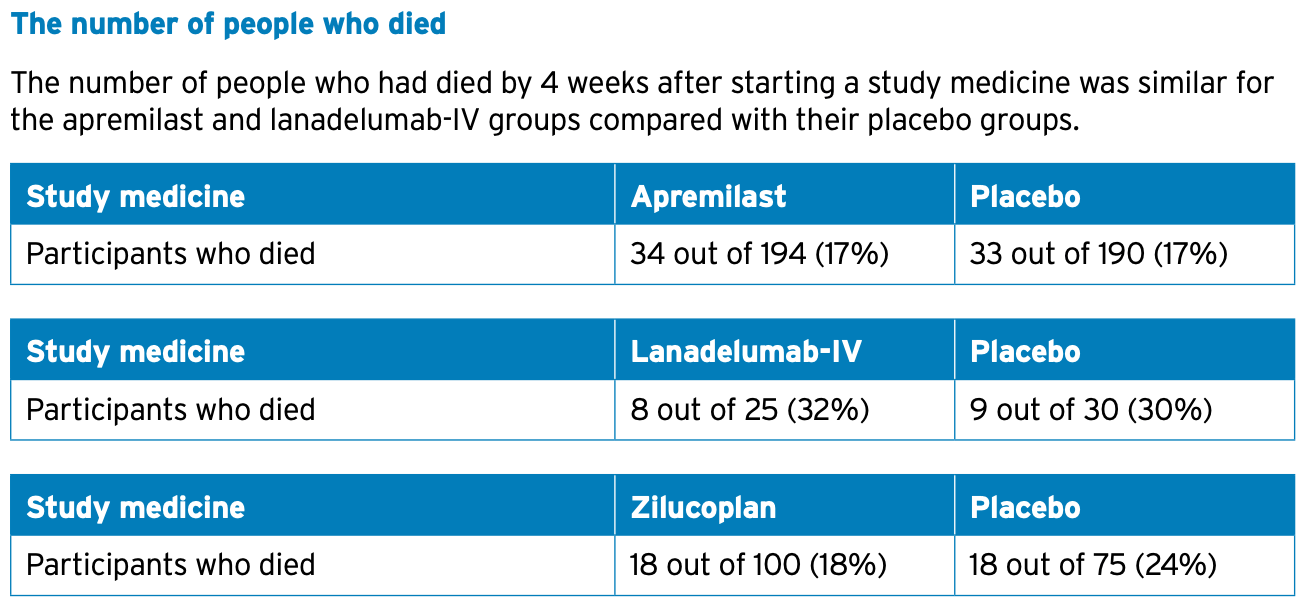
• died.
Overall, researchers found that none of the..
amgen