Favipiravir for the Treatment of Coronavirus Disease 2019 Pneumonia; a Propensity Score-matched Cohort Study
et al., Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.08.011, Nov 2021 (preprint)
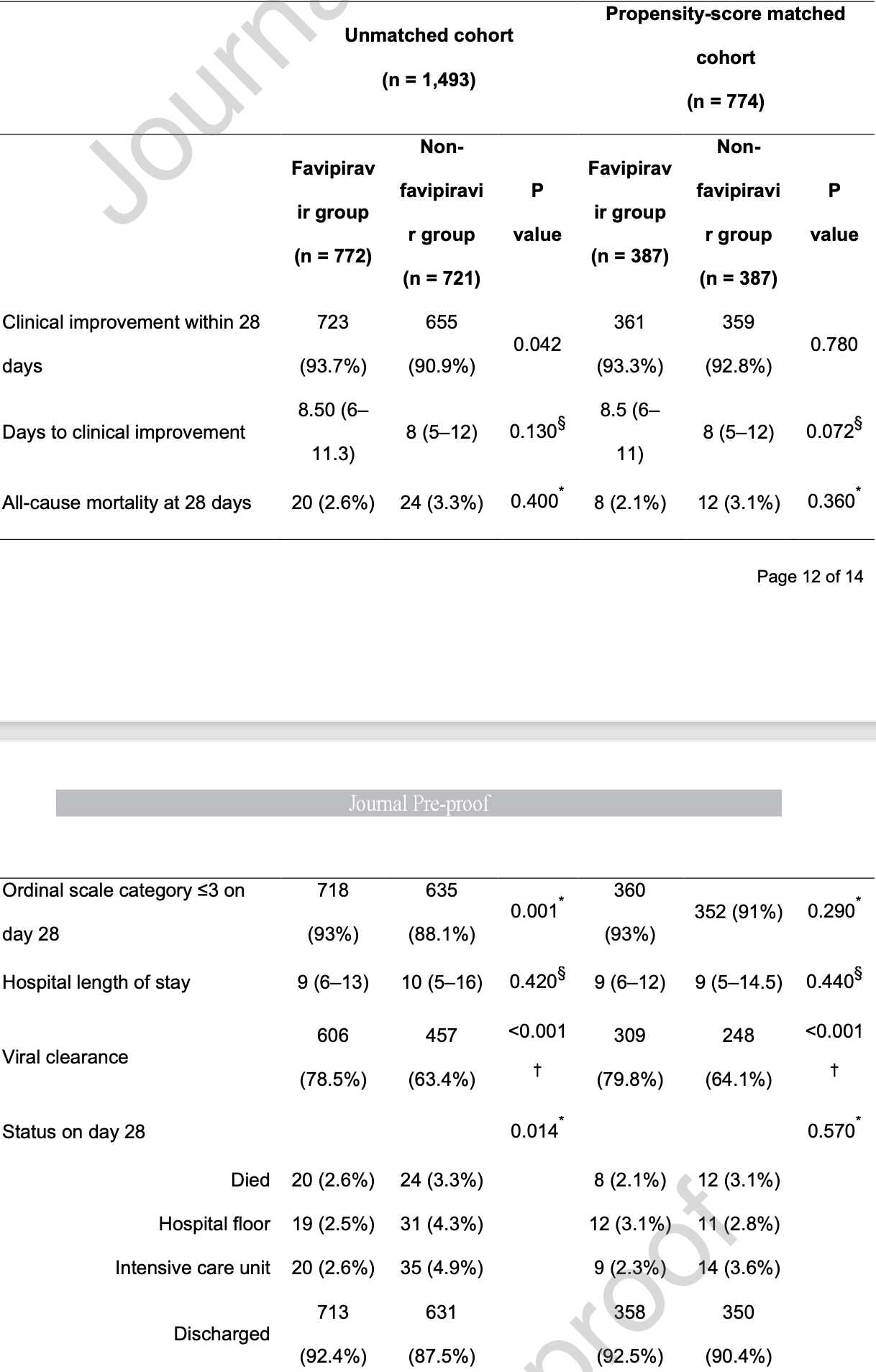
PSM retrospective with 1,493 patients, showing significantly improved viral clearance with favipiravir. There were no significant differences in clinical improvement or mortality. Mortality was lower (2.1% vs 3.1%), without statistical significance with the small number of events.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
|
risk of death, 33.3% lower, RR 0.67, p = 0.50, treatment 8 of 387 (2.1%), control 12 of 387 (3.1%), NNT 97, propensity score matching, day 28.
|
|
risk of no clinical improvement, 2.2% higher, RR 1.02, p = 0.73, treatment 26 of 387 (6.7%), control 28 of 387 (7.2%), NNT 194, adjusted per study, inverted to make RR<1 favor treatment, day 28, Cox proportional hazards, propensity score matching, primary outcome.
|
|
days to clinical improvement, 6.2% higher, relative time 1.06, p = 0.07, treatment 387, control 387, propensity score matching.
|
|
risk of no viral clearance, 43.9% lower, RR 0.56, p < 0.001, treatment 78 of 387 (20.2%), control 139 of 387 (35.9%), NNT 6.3, propensity score matching.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Alattar et al., 30 Nov 2021, retrospective, Qatar, peer-reviewed, median age 46.0, 25 authors, study period 23 May, 2020 - 18 July, 2020, average treatment delay 5.0 days.
Contact: aomrani@hamad.qa.
Favipiravir for the treatment of coronavirus disease 2019 pneumonia; a propensity score-matched cohort study
Journal of Infection and Public Health, doi:10.1016/j.jiph.2022.08.011
This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical approval The study was approved by the Institutional Review Board at Hamad Medical Corporation (MRC-01-20-994), with a waiver of informed consent.
Funding The publication of this article was funded by the Qatar National Library. J o u r n a l P r e -p r o o f
Declaration of Interest
None
References
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the Treatment of Covid-19 -Final Report, N Engl J Med, doi:10.1056/NEJMoa2007764
Cevik, Kuppalli, Kindrachuk, Peiris, Virology, transmission, and pathogenesis of SARS-CoV-2, BMJ, doi:10.1136/bmj.m3862
Doi, Hibino, Hase, Yamamoto, Kasamatsu et al., A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19, Antimicrob Agents Chemother, doi:10.1128/AAC.01897-20
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci, doi:10.2183/pjab.93.027
Ivashchenko, Dmitriev, Vostokova, Azarova, Blinow et al., AVIFAVIR for Treatment of Patients with Moderate COVID-19: Interim Results of a Phase II/III Multicenter Randomized Clinical Trial, Clin Infect Dis, doi:10.1093/cid/ciaa1176
J O U R N A L P R E, -p r o o f
Kumagai, Murakawa, Hasunuma, Aso, Yuji et al., Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults, Int J Clin Pharmacol Ther, doi:10.5414/cp202388
Lagocka, Dziedziejko, Kłos, Pawlik, Favipiravir in Therapy of Viral Infections, J Clin Med, doi:10.3390/jcm10020273
Manabe, Kambayashi, Akatsu, Kudo, Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis, BMC Infect Dis, doi:10.1186/s12879-021-06164-x
Özlüşen, Kozan, Akcan, Kalender, Yaprak et al., Effectiveness of favipiravir in COVID-19: a live systematic review, Eur J Clin Microbiol Infect Dis, doi:10.1007/s10096-021-04307-1
DOI record:
{
"DOI": "10.1016/j.jiph.2022.08.011",
"ISSN": [
"1876-0341"
],
"URL": "http://dx.doi.org/10.1016/j.jiph.2022.08.011",
"alternative-id": [
"S187603412200212X"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Favipiravir for the Treatment of Coronavirus Disease 2019 Pneumonia; a Propensity Score-matched Cohort Study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "Journal of Infection and Public Health"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.jiph.2022.08.011"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2022 The Author(s). Published by Elsevier Ltd on behalf of King Saud Bin Abdulaziz University for Health Sciences."
}
],
"author": [
{
"affiliation": [],
"family": "Alattar",
"given": "Rand A.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Abdalla",
"given": "Shiema",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdallah",
"given": "Tasneem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kazman",
"given": "Rashid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qadmour",
"given": "Aseelah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ibrahim",
"given": "Tawheeda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alhariri",
"given": "Bassem",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shaar",
"given": "Shahd H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bajwa",
"given": "Abeer",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alimam",
"given": "Abeir",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qazi",
"given": "Rabia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ben Abid",
"given": "Fatma",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daghfal",
"given": "Joanne",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eldeeb",
"given": "Ali",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shukri",
"given": "Kinda",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Elsayed",
"given": "Ahmed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rustom",
"given": "Fatima",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alsamawi",
"given": "Musaed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdelmajid",
"given": "Alaaeldin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Basulto",
"given": "Miguel A.P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cobian",
"given": "Armando A.R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abukhattab",
"given": "Mohamed",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alkhal",
"given": "Abdullatif",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Almaslamani",
"given": "Muna A.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Omrani",
"given": "Ali S.",
"sequence": "additional"
}
],
"container-title": "Journal of Infection and Public Health",
"container-title-short": "Journal of Infection and Public Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2022,
8,
27
]
],
"date-time": "2022-08-27T23:56:42Z",
"timestamp": 1661644602000
},
"deposited": {
"date-parts": [
[
2022,
8,
27
]
],
"date-time": "2022-08-27T23:56:51Z",
"timestamp": 1661644611000
},
"indexed": {
"date-parts": [
[
2022,
8,
28
]
],
"date-time": "2022-08-28T00:12:51Z",
"timestamp": 1661645571683
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
8
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
1
]
],
"date-time": "2022-08-01T00:00:00Z",
"timestamp": 1659312000000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 21,
"start": {
"date-parts": [
[
2022,
8,
22
]
],
"date-time": "2022-08-22T00:00:00Z",
"timestamp": 1661126400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S187603412200212X?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S187603412200212X?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"prefix": "10.1016",
"published": {
"date-parts": [
[
2022,
8
]
]
},
"published-print": {
"date-parts": [
[
2022,
8
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.2183/pjab.93.027",
"article-title": "Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase",
"author": "Furuta",
"doi-asserted-by": "crossref",
"first-page": "449",
"journal-title": "Proc Jpn Acad Ser B Phys Biol Sci",
"key": "10.1016/j.jiph.2022.08.011_bib1",
"volume": "93",
"year": "2017"
},
{
"DOI": "10.1007/s10096-021-04307-1",
"article-title": "Effectiveness of favipiravir in COVID-19: a live systematic review",
"author": "Özlüşen",
"doi-asserted-by": "crossref",
"first-page": "2575",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "10.1016/j.jiph.2022.08.011_bib2",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the Treatment of Covid-19 — Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2022.08.011_bib3",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06164-x",
"article-title": "Favipiravir for the treatment of patients with COVID-19: a systematic review and meta-analysis",
"author": "Manabe",
"doi-asserted-by": "crossref",
"first-page": "489",
"journal-title": "BMC Infect Dis",
"key": "10.1016/j.jiph.2022.08.011_bib4",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1136/bmj.m3862",
"article-title": "Virology, transmission, and pathogenesis of SARS-CoV-2",
"author": "Cevik",
"doi-asserted-by": "crossref",
"first-page": "m3862",
"journal-title": "BMJ",
"key": "10.1016/j.jiph.2022.08.011_bib5",
"volume": "371",
"year": "2020"
},
{
"DOI": "10.3390/jcm10020273",
"article-title": "Favipiravir in Therapy of Viral Infections",
"author": "Lagocka",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "J Clin Med",
"key": "10.1016/j.jiph.2022.08.011_bib6",
"volume": "10",
"year": "2021"
},
{
"article-title": "A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19",
"author": "Doi",
"first-page": "64",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.jiph.2022.08.011_bib7",
"year": "2020"
},
{
"article-title": "Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19",
"author": "RECOVERY",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/j.jiph.2022.08.011_bib8",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(21)00676-0",
"article-title": "Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "Lancet",
"key": "10.1016/j.jiph.2022.08.011_bib9",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1176",
"article-title": "AVIFAVIR for Treatment of Patients with Moderate COVID-19: Interim Results of a Phase II/III Multicenter Randomized Clinical Trial",
"author": "Ivashchenko",
"doi-asserted-by": "crossref",
"first-page": "531",
"journal-title": "Clin Infect Dis",
"key": "10.1016/j.jiph.2022.08.011_bib10",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.5414/CP202388",
"article-title": "Lack of effect of favipiravir, a novel antiviral agent, on QT interval in healthy Japanese adults",
"author": "Kumagai",
"doi-asserted-by": "crossref",
"first-page": "866",
"journal-title": "Int J Clin Pharmacol Ther",
"key": "10.1016/j.jiph.2022.08.011_bib11",
"volume": "53",
"year": "2015"
}
],
"reference-count": 11,
"references-count": 11,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S187603412200212X"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Public Health, Environmental and Occupational Health",
"General Medicine"
],
"subtitle": [],
"title": "Favipiravir for the Treatment of Coronavirus Disease 2019 Pneumonia; a Propensity Score-matched Cohort Study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}