Favipiravir Experience in COVID-19 Patients at a Tertiary Center Intensive Care Unit
, S., SiSli Etfal Hastanesi Tip Bulteni / The Medical Bulletin of Sisli Hospital, doi:10.14744/SEMB.2021.35902, NCT04645433, Jun 2022
Retrospective 100 ICU patients in Turkey, showing improved survival with favipiravir vs. lopinavir/ritonavir.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
This study is excluded in the after exclusion results of meta-analysis:
very late stage, ICU patients.
|
risk of death, 16.2% lower, RR 0.84, p = 0.38, treatment 57 of 85 (67.1%), control 12 of 15 (80.0%), NNT 7.7.
|
|
risk of mechanical ventilation, 10.3% lower, RR 0.90, p = 0.75, treatment 61 of 85 (71.8%), control 12 of 15 (80.0%), NNT 12.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Acar Sevinc et al., 28 Jun 2022, retrospective, Turkey, peer-reviewed, mean age 65.6, 1 author, study period 10 March, 2020 - 10 May, 2020, this trial compares with another treatment - results may be better when compared to placebo, trial NCT04645433 (history).
Contact: sultanacar34@hotmail.com.
Favipiravir Experience in COVID-19 Patients at a Tertiary Center Intensive Care Unit
SiSli Etfal Hastanesi Tip Bulteni / The Medical Bulletin of Sisli Hospital, doi:10.14744/semb.2021.35902
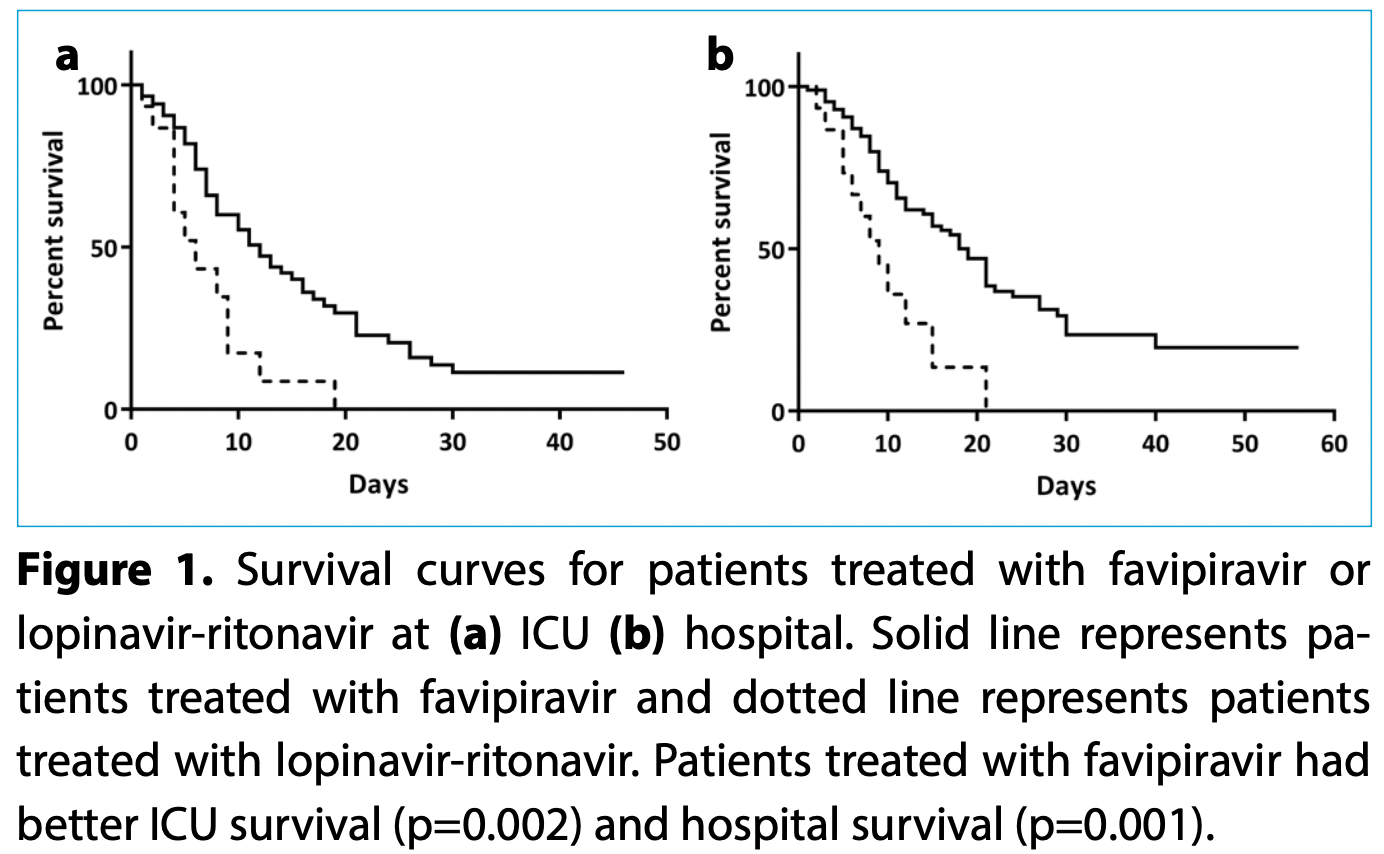
is the disease caused by severe acute respira- tory syndrome coronavirus 2 (SARS-CoV-2) infection and may present in different clinical scenarios: About 80% of patients present with mild, 13.8% present with severe disease, and 6.1% with critical disease. [1] According to the guidelines published by the Ministry of Health in Turkey, patients with severe and critical disease were advised to be admitted to intensive care unit (ICU). [2] Turkey diagnosed Objectives: The aim of this study was to compare intensive care unit (ICU) and overall hospital mortality in patients treated with favipiravir and lopinavir-ritonavir for COVID-19. Methods: Data were collected retrospectively between March 10 and May 10, 2020, from patients' records admitted to ICU due to COVID-19. Laboratory data, clinical characteristics, ICU and hospital mortality, ICU and hospital length of stay were compared in patients treated with favipiravir and lopinavir-ritonavir. Results: A total of 100 patients' data were investigated. Favipiravir was used as the treatment for 85% of patients, with the rest treated with lopinavir-ritonavir. Clinical and laboratory data of both antiviral treatment groups were similar. Length of hospital stay was 16 (9-24) days with favipiravir and 8.5 (5-12.5) days with lopinavir-ritonavir (p=0.002). Length of ICU stay for favipiravir and lopinavir-ritonavir groups were 8 (5-15) days and 4 (3-9) days, respectively (p=0.011). ICU mortality was 65.9% for the favipiravir and 80% for lopinavir-ritonavir (p=0.002). Hospital mortality for favipiravir and lopinavir-ritonavir was 67.1% and 80%, respectively (p=0.001).
Conclusion: The mortality in patients treated with favipiravir was less than patients treated with lopinavir-ritonavir. Favipiravir needs more attention and trials for its effect to be confirmed.
References
Bhatraju, Ghassemieh, Nichols, Kim, Jerome et al., Covid-19 in critically Ill patients in the Seattle regioncase series, N Engl J Med, doi:10.1056/NEJMoa2004500
Cai, Yang, Liu, Chen, Shu et al., Experimental treatment with favipiravir for COVID-19: An open-label control study, Engineering, doi:10.1016/j.eng.2020.03.007
Chen, Liang, Li, Guo, Fei et al., Mental health care for medical staff in China during the COVID-19 outbreak, Lancet Psychiatry, doi:10.1016/S2215-0366(20)30078-X
Du, Chen, Favipiravir: Pharmacokinetics and concerns about clinical trials for 2019-nCoV infection, Clin Pharmacol Ther, doi:10.1002/cpt.1844
Furuta, Gowen, Takahashi, Shiraki, Smee et al., Favipiravir (T-705), a novel viral RNA polymerase inhibitor, Antiviral Res, doi:10.1016/j.antiviral.2013.09.015
Grasselli, Zangrillo, Zanella, Antonelli, Cabrini et al., Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy, JAMA, doi:10.1001/jama.2020.5394
Ilhan, Altuntas, Optimum management of COVID-19 in the geriatric population: The need for a comprehensive assessment
Kupferschmidt, Cohen, WHO launches global megatrial of the four most promising coronavirus treatments
Mitra, Fergusson, Lloyd-Smith, Wormsbecker, Foster et al., Baseline characteristics and outcomes of patients with COVID-19 admitted to intensive care units in Vancouver, Canada: a case series, CMAJ, doi:10.1503/cmaj.200794
Pascarella, Strumia, Piliego, Bruno, Buono et al., COVID-19 diagnosis and management: a comprehensive review, J Intern Med, doi:10.1111/joim.13091
Shiraki, Daikoku, Favipiravir, an anti-influenza drug against life-threatening RNA virus infections, Pharmacol Ther, doi:10.1016/j.pharmthera.2020.107512
Stringer, Puskarich, Kenes, Dickson, COVID-19: The uninvited guest in the intensive care unit -implications for pharmacotherapy, Pharmacotherapy, doi:10.1002/phar.2394
Wang, Hu, Hu, Zhu, Liu et al., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirusinfected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Yamamoto, Matsuyama, Hoshino, Yamamoto, Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro, bioRxiv, doi:10.1101/2020.04.06.026476
Zheng, Sun, Xu, Pan, Zhang et al., Clinical characteristics of 34 COVID-19 patients admitted to intensive care unit in Hangzhou, China, J Zhejiang Univ Sci B, doi:10.1631/jzus.B2000174
DOI record:
{
"DOI": "10.14744/semb.2021.35902",
"ISSN": [
"1302-7123"
],
"URL": "http://dx.doi.org/10.14744/SEMB.2021.35902",
"author": [
{
"affiliation": [],
"family": "Acar Sevinc",
"given": "Sultan",
"sequence": "first"
}
],
"container-title": "SiSli Etfal Hastanesi Tip Bulteni / The Medical Bulletin of Sisli Hospital",
"container-title-short": "Sisli Etfal",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
9,
22
]
],
"date-time": "2021-09-22T07:37:37Z",
"timestamp": 1632296257000
},
"deposited": {
"date-parts": [
[
2021,
9,
22
]
],
"date-time": "2021-09-22T07:37:39Z",
"timestamp": 1632296259000
},
"indexed": {
"date-parts": [
[
2022,
3,
29
]
],
"date-time": "2022-03-29T14:01:08Z",
"timestamp": 1648562468806
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021
]
]
},
"member": "5966",
"original-title": [],
"prefix": "10.14744",
"published": {
"date-parts": [
[
2021
]
]
},
"published-online": {
"date-parts": [
[
2021
]
]
},
"published-print": {
"date-parts": [
[
2021
]
]
},
"publisher": "Kare Publishing",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://sislietfaltip.org/jvi.aspx?un=SETB-35902&volume="
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subtitle": [],
"title": "Favipiravir Experience in COVID-19 Patients at a Tertiary Center Intensive Care Unit",
"type": "journal-article"
}