Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial
et al., eClinicalMedicine, doi:10.1016/j.eclinm.2021.101169, NCT04623021, Nov 2021
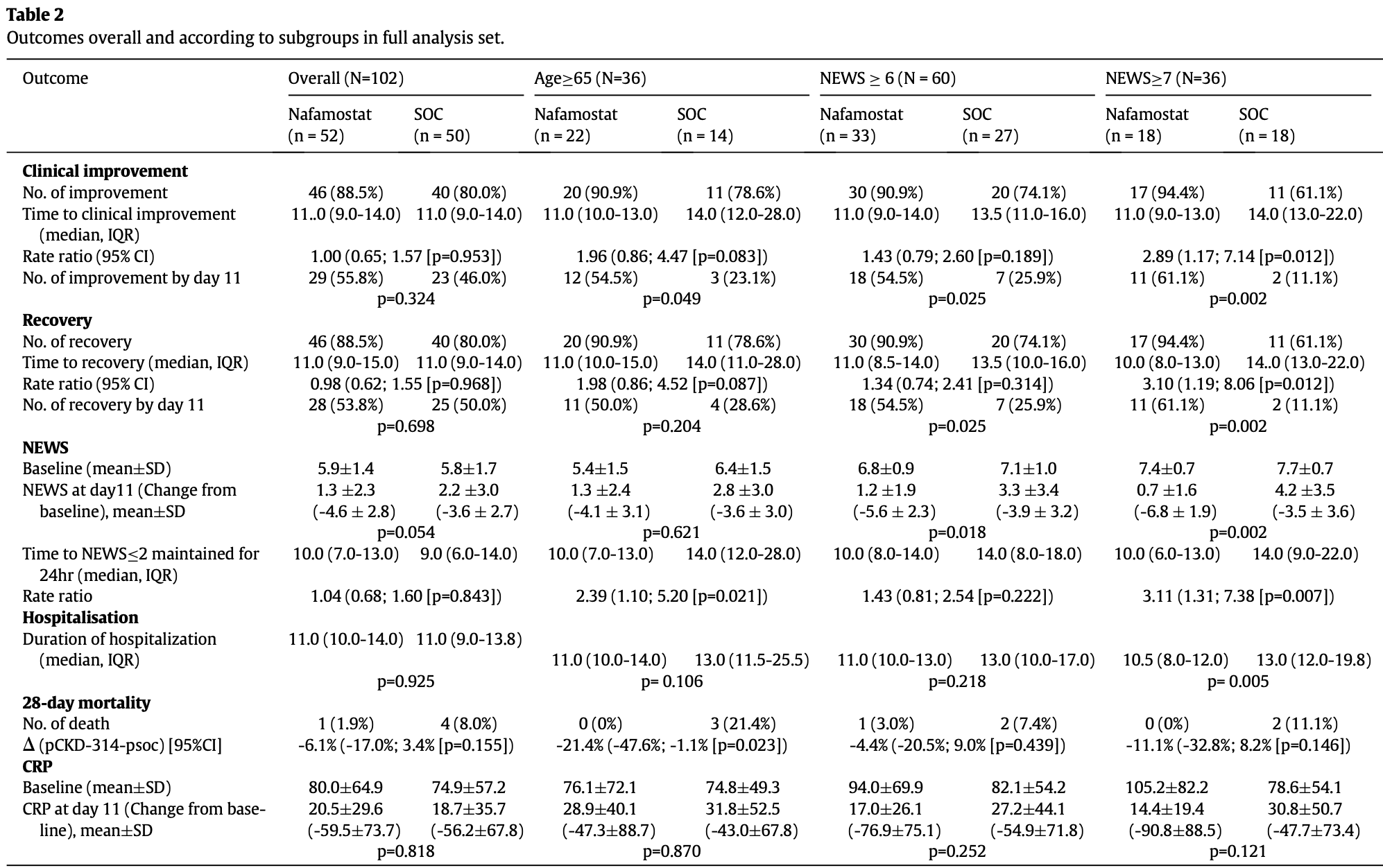
RCT 104 hospitalized patients with moderate to severe COVID-19 pneumonia showing no significant difference in the primary endpoint of time to clinical improvement with nafamostat. However, in patients with baseline National Early Warning Score (NEWS) ≥7, nafamostat treatment significantly shortened time to clinical improvement and recovery. Patients in the nafamostat group with NEWS ≥7 also had higher recovery rates and significantly reduced NEWS scores by day 11.
Study covers TMPRSS2 inhibitors and nafamostat.
|
risk of death, 76.0% lower, RR 0.24, p = 0.20, treatment 1 of 52 (1.9%), control 4 of 50 (8.0%), NNT 16.
|
|
risk of no improvement, 42.3% lower, RR 0.58, p = 0.28, treatment 6 of 52 (11.5%), control 10 of 50 (20.0%), NNT 12.
|
|
risk of no recovery, 42.3% lower, RR 0.58, p = 0.28, treatment 6 of 52 (11.5%), control 10 of 50 (20.0%), NNT 12.
|
|
risk of no recovery, 40.9% lower, RR 0.59, p = 0.09, treatment mean 1.3 (±2.3) n=52, control mean 2.2 (±3.0) n=50, relative NEWS score at day 11, day 11.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Zhuravel et al., 30 Nov 2021, Randomized Controlled Trial, placebo-controlled, Russia, peer-reviewed, mean age 58.6, 7 authors, study period 25 September, 2020 - 14 November, 2020, average treatment delay 10.0 days, trial NCT04623021 (history).
Contact: zhsergey5@gmail.com.
Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial
eClinicalMedicine, doi:10.1016/j.eclinm.2021.101169
Background: Nafamostat, a serine protease inhibitor, has been used for the treatment of disseminated intravascular coagulation and pancreatitis. In vitro studies and clinical reports suggest its beneficial effect in the treatment of COVID-19 pneumonia. Methods: This phase 2 open-label, randomised, multicentre, controlled trial evaluated nafamostat (4.8 mg/kg/ day) plus standard-of-care (SOC) in hospitalised patients with COVID-19 pneumonia (i.e., those requiring nasal high-flow oxygen therapy and/or non-invasive mechanical ventilation). The primary outcome was the time to clinical improvement. Key secondary outcomes included the time to recovery, rates of recovery and National Early Warning Score (NEWS). The trial is registered with ClinicalTrials.gov Identifier: NCT04623021. Findings: A total of 104 patients, mean age 58.6 years were enrolled in 13 clinical centres in Russia between 25/9/2020 and 14/11/2020 and randomised to nafamostat plus SOC (n=53) or SOC alone (n=51). There was no significant difference in time to clinical improvement (primary endpoint) between the nafamostat and SOC groups (median 11 [interquartile range (IQR) 9 to 14) vs 11 [IQR 9 to 14] days; Rate Ratio [RR; the ratio for clinical improvement], 1.00; 95% CI, 0.65 to 1.57; p=0.953). In 36 patients with baseline NEWS 7, nafamostat was superior to SOC alone in median time to clinical improvement (11 vs 14 days; RR, 2.89; 95% CI, 1.17 to 7.14; p=0.012). Patients receiving nafamostat in this subgroup had a significantly higher recovery rate compared with SOC alone (61.1% (11/18) vs 11.1 % (2/18) by Day 11, p=0.002). The 28-day mortality was 1.9% (1/52) for nafamostat and 8.0% (4/50) for SOC (95% CI, p=0.155). No case of COVID-19 related serious adverse events leading to death was recorded in the patients receiving nafamostat. Interpretation: Our study found no significant difference in time to clinical improvement between the nafamostat and SOC groups, but a shorter median time to clinical improvement in a small group of high-risk COVID-19 patients requiring oxygen treatment. To assess the efficacy further, a larger Phase 3 clinical trial is warranted.
Author Contributions SVZ was the coordinating investigator and OKK, OOB, AIG and BMG were principal investigators of the study. SVZ, OKK, OOB, AIG and BMG contributed to the implementation of the study and interpretation of the data. SVZ contributed revising the manuscript for important intellectual content and had final responsibility for the decision to submit for publication with agreement of all authors. SK and KYH were involved in the study design, analysis and interpretation of the data, and contributed to writing the manuscript. All authors reviewed and approved the final manuscript.
Declaration of Competing Interest SK and KYH are both employees of CKD Pharm. The Investigators declare that the research was supported by a grant from CKD Pharm, South Korea, and have no other relationships that could be construed as a potential conflict of interest.
Data sharing statement The datasets generated for this study and relevant documents including clinical study documents (e.g., study report, study protocol, statistical analysis plan) will be made available to be shared after publication of the manuscript in a peer-reviewed journal and if regulatory activities are complete, on requests directed to the corresponding author. Prior to providing data, documents will be examined, and, if necessary, redacted and the data will be de-identified, to protect the personal data of study participants and personnel, and to respect the boundaries of the informed consent of the study..
References
Akizawa, Koshikawa, Ota, Nafamostat mesilate: a regional anticoagulant for hemodialysis in patients at high risk for bleeding, Nephron
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19 -Final Report, N Engl J Med
Cao, Wang, Wen, A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19, N Engl J Med
Cavalcanti, Zampieri, Rosa, Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19, N Engl J Med
Guan, Ni, Hu, Clinical Characteristics of Coronavirus Disease 2019 in China, N Engl J Med
Han, Kim, Kim, Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation, J Korean Med Sci
Hoffmann, Kleine-Weber, Schroeder, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell
Hoffmann, Schroeder, Kleine-Weber, Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for Covid-19, Antimicrob Agents Chemother, doi:10.1128/AAC.00754-20
Horby, Lim, Emberson, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med
Interim Guidelines, Prevention, diagnosis and treatment of a new coronavirus infection (COVID-19), Ministry of Health of the Russian Federation
Jang, Rhee, Three cases of treatment with nafamostat in elderly patients with Covid-19 pneumonia who need oxygen therapy, Int J Infect Dis
Ko, Jeon, Ryu, Comparative analysis of antiviral efficacy of FDAapproved drugs against SARS-CoV-2 in human lung cells, J Med Virol, doi:10.1002/jmv.26397
Minakata, Fujiwara, Ikeda, Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematological malignancies, Int J Hematol
O'driscoll, Santos, Wang, Age-specific mortality and immunity patterns of SARS-CoV-2, Nature
Polyakova, Kocks, Udalova, Initial economic damage from the COVID-19 pandemic in the United States is more widespread across ages and geographies than initial mortality impacts, Proc Natl Acad Sci
Self, Semler, Leither, Effect of Hydroxychloroquine on clinical status at 14 days in hospitalized patients with Covid-19: a randomized clinical trial, JAMA
Tagawa, Protease inhibitor nafamostat mesilate attenuates complement activation and improves function of xenografts in a discordant lung perfusion model, Xenotransplantation
Takahashi, Yoneda, Koba, Potential mechanisms of nafamostat therapy for severe Covid-19 pneumonia with disseminated intravascular coagulation, Int J Infect Dis
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Research
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention, JAMA
Yamamoto, Kiso, Sakai-Tagawa, The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner, Viruses
Yang, Han, Kim, Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage, Renal Fail
Zhou, Yang, Wang, A pneumonia outbreak associated with a new coronavirus of probable bat origin, Nature
DOI record:
{
"DOI": "10.1016/j.eclinm.2021.101169",
"ISSN": [
"2589-5370"
],
"URL": "http://dx.doi.org/10.1016/j.eclinm.2021.101169",
"alternative-id": [
"S2589537021004491"
],
"article-number": "101169",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "eClinicalMedicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.eclinm.2021.101169"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 The Authors. Published by Elsevier Ltd."
}
],
"author": [
{
"affiliation": [],
"family": "Zhuravel",
"given": "Sergey V",
"sequence": "first"
},
{
"affiliation": [],
"family": "Khmelnitskiy",
"given": "Oleg K",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Burlaka",
"given": "Oleg O",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gritsan",
"given": "Alexey I",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Goloshchekin",
"given": "Boris M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kim",
"given": "Seieun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6079-8259",
"affiliation": [],
"authenticated-orcid": false,
"family": "Hong",
"given": "Ka Young",
"sequence": "additional"
}
],
"container-title": "eClinicalMedicine",
"container-title-short": "eClinicalMedicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
10,
26
]
],
"date-time": "2021-10-26T22:19:41Z",
"timestamp": 1635286781000
},
"deposited": {
"date-parts": [
[
2022,
11,
3
]
],
"date-time": "2022-11-03T19:56:55Z",
"timestamp": 1667505415000
},
"indexed": {
"date-parts": [
[
2025,
4,
10
]
],
"date-time": "2025-04-10T08:10:37Z",
"timestamp": 1744272637177
},
"is-referenced-by-count": 61,
"issued": {
"date-parts": [
[
2021,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
11,
1
]
],
"date-time": "2021-11-01T00:00:00Z",
"timestamp": 1635724800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
10,
6
]
],
"date-time": "2021-10-06T00:00:00Z",
"timestamp": 1633478400000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021004491?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589537021004491?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "101169",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
11
]
]
},
"published-print": {
"date-parts": [
[
2021,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1038/s41586-020-2012-7",
"article-title": "A pneumonia outbreak associated with a new coronavirus of probable bat origin",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "270",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2021.101169_bib0001",
"volume": "579",
"year": "2020"
},
{
"DOI": "10.1038/s41586-020-2918-0",
"article-title": "Age-specific mortality and immunity patterns of SARS-CoV-2",
"author": "O'Driscoll",
"doi-asserted-by": "crossref",
"first-page": "140",
"journal-title": "Nature",
"key": "10.1016/j.eclinm.2021.101169_bib0002",
"volume": "590",
"year": "2021"
},
{
"DOI": "10.1073/pnas.2014279117",
"article-title": "Initial economic damage from the COVID-19 pandemic in the United States is more widespread across ages and geographies than initial mortality impacts",
"author": "Polyakova",
"doi-asserted-by": "crossref",
"first-page": "27934",
"journal-title": "Proc Natl Acad Sci",
"key": "10.1016/j.eclinm.2021.101169_bib0003",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.2648",
"article-title": "Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention",
"author": "Wu",
"doi-asserted-by": "crossref",
"first-page": "1239",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.101169_bib0004",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2002032",
"article-title": "Clinical Characteristics of Coronavirus Disease 2019 in China",
"author": "Guan",
"doi-asserted-by": "crossref",
"first-page": "1708",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101169_bib0005",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"article-title": "SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "271",
"journal-title": "Cell",
"key": "10.1016/j.eclinm.2021.101169_bib0006",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1128/AAC.00754-20",
"article-title": "Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for Covid-19",
"author": "Hoffmann",
"doi-asserted-by": "crossref",
"first-page": "e00754",
"issue": "6",
"journal-title": "Antimicrob Agents Chemother",
"key": "10.1016/j.eclinm.2021.101169_bib0007",
"volume": "64",
"year": "2020"
},
{
"DOI": "10.3390/v12060629",
"article-title": "The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner",
"author": "Yamamoto",
"doi-asserted-by": "crossref",
"first-page": "629",
"journal-title": "Viruses",
"key": "10.1016/j.eclinm.2021.101169_bib0008",
"volume": "12",
"year": "2020"
},
{
"article-title": "Comparative analysis of antiviral efficacy of FDA-approved drugs against SARS-CoV-2 in human lung cells",
"author": "Ko",
"journal-title": "J Med Virol",
"key": "10.1016/j.eclinm.2021.101169_bib0009",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Research",
"key": "10.1016/j.eclinm.2021.101169_bib0010",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.22240",
"article-title": "Effect of Hydroxychloroquine on clinical status at 14 days in hospitalized patients with Covid-19: a randomized clinical trial",
"author": "Self",
"doi-asserted-by": "crossref",
"first-page": "2165",
"journal-title": "JAMA",
"key": "10.1016/j.eclinm.2021.101169_bib0011",
"volume": "324",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2019014",
"article-title": "Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19",
"author": "Cavalcanti",
"doi-asserted-by": "crossref",
"first-page": "2041",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101169_bib0012",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"first-page": "1787",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101169_bib0013",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19 - Final Report",
"author": "Beigel",
"doi-asserted-by": "crossref",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101169_bib0014",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in Hospitalized Patients with Covid-19",
"author": "Horby",
"doi-asserted-by": "crossref",
"first-page": "693",
"journal-title": "N Engl J Med",
"key": "10.1016/j.eclinm.2021.101169_bib0015",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1159/000187357",
"article-title": "Nafamostat mesilate: a regional anticoagulant for hemodialysis in patients at high risk for bleeding",
"author": "Akizawa",
"doi-asserted-by": "crossref",
"first-page": "376",
"journal-title": "Nephron",
"key": "10.1016/j.eclinm.2021.101169_bib0016",
"volume": "64",
"year": "1993"
},
{
"DOI": "10.3109/08860220903180616",
"article-title": "Superior outcome of nafamostat mesilate as an anticoagulant in patients undergoing maintenance hemodialysis with intracerebral hemorrhage",
"author": "Yang",
"doi-asserted-by": "crossref",
"first-page": "668",
"journal-title": "Renal Fail",
"key": "10.1016/j.eclinm.2021.101169_bib0017",
"volume": "31",
"year": "2009"
},
{
"DOI": "10.3346/jkms.2011.26.7.945",
"article-title": "Use of nafamostat mesilate as an anticoagulant during extracorporeal membrane oxygenation",
"author": "Han",
"doi-asserted-by": "crossref",
"first-page": "945",
"journal-title": "J Korean Med Sci",
"key": "10.1016/j.eclinm.2021.101169_bib0018",
"volume": "26",
"year": "2011"
},
{
"DOI": "10.1007/s12185-018-02567-w",
"article-title": "Comparison of gabexate mesilate and nafamostat mesilate for disseminated intravascular coagulation associated with hematological malignancies",
"author": "Minakata",
"doi-asserted-by": "crossref",
"first-page": "141",
"journal-title": "Int J Hematol",
"key": "10.1016/j.eclinm.2021.101169_bib0019",
"volume": "109",
"year": "2019"
},
{
"DOI": "10.1111/j.1399-3089.2011.00650.x",
"article-title": "Protease inhibitor nafamostat mesilate attenuates complement activation and improves function of xenografts in a discordant lung perfusion model",
"author": "Tagawa",
"doi-asserted-by": "crossref",
"first-page": "315",
"journal-title": "Xenotransplantation",
"key": "10.1016/j.eclinm.2021.101169_bib0020",
"volume": "18",
"year": "2011"
},
{
"DOI": "10.1016/j.ijid.2020.05.072",
"article-title": "Three cases of treatment with nafamostat in elderly patients with Covid-19 pneumonia who need oxygen therapy",
"author": "Jang",
"doi-asserted-by": "crossref",
"first-page": "500",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.eclinm.2021.101169_bib0021",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1016/j.ijid.2020.10.093",
"article-title": "Potential mechanisms of nafamostat therapy for severe Covid-19 pneumonia with disseminated intravascular coagulation",
"author": "Takahashi",
"doi-asserted-by": "crossref",
"first-page": "529",
"journal-title": "Int J Infect Dis",
"key": "10.1016/j.eclinm.2021.101169_bib0022",
"volume": "102",
"year": "2021"
},
{
"key": "10.1016/j.eclinm.2021.101169_bib0023",
"series-title": "WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2021.101169_bib0024",
"volume": "7",
"year": "2020"
},
{
"key": "10.1016/j.eclinm.2021.101169_bib0025",
"series-title": "National Early Warning Score (NEWS): Standardising the assessment of acute-illness severity in the NHS",
"year": "2012"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589537021004491"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"special_numbering": "C",
"subject": [],
"subtitle": [],
"title": "Nafamostat in hospitalized patients with moderate to severe COVID-19 pneumonia: a randomised Phase II clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"volume": "41"
}
zhuravel