Azvudine efficacy in reducing mortality in COVID-19 patients
et al., European Journal of Medical Research, doi:10.1186/s40001-024-02220-9, Dec 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
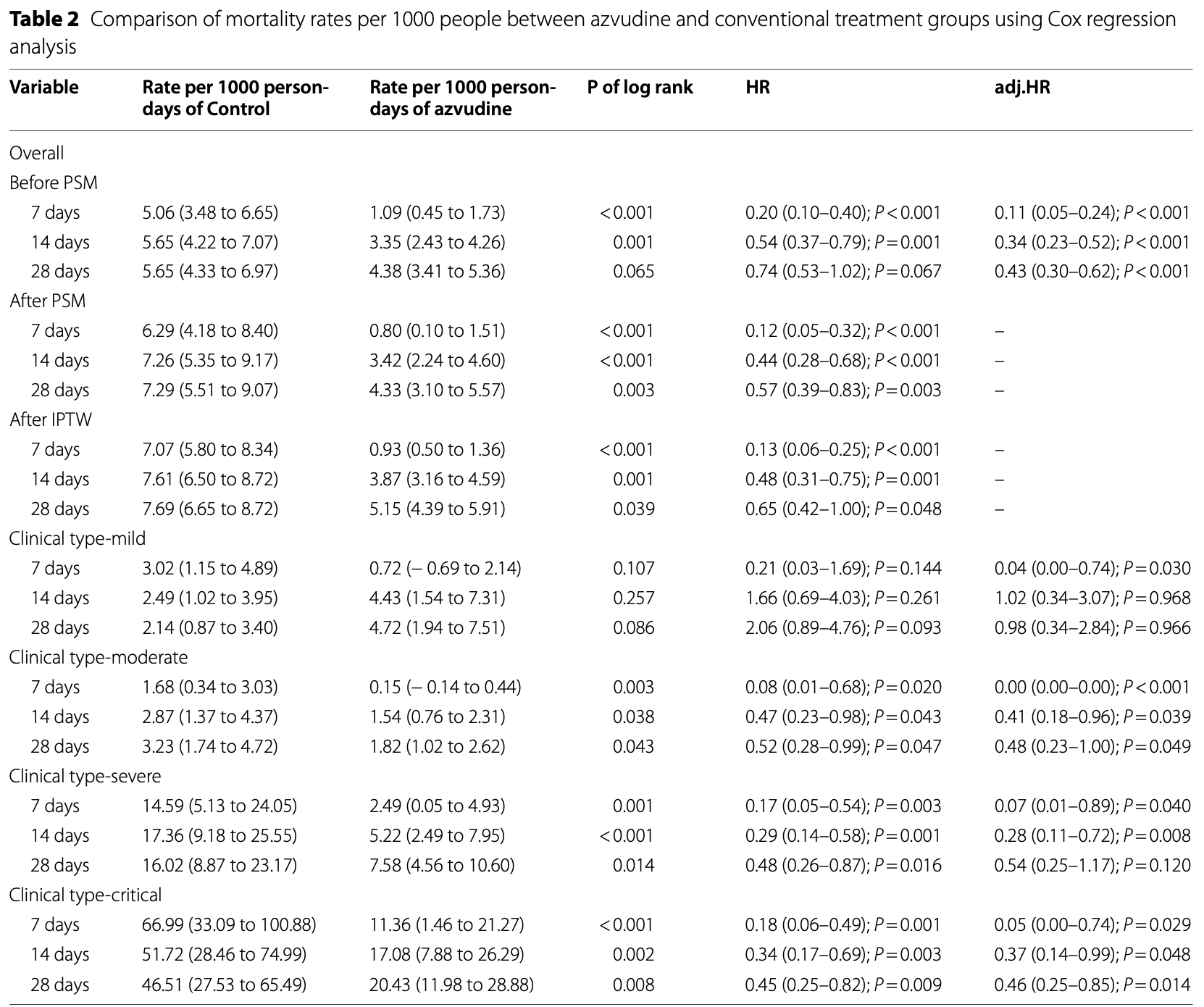
Retrospective 2,862 hospitalized COVID-19 patients in China showing lower mortality with azvudine treatment, with greater efficacy for severe and critical patients.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 35.0% lower, HR 0.65, p = 0.048, treatment 1,490, control 1,372, propensity score weighting, day 28.
|
|
risk of death, 43.0% lower, HR 0.57, p = 0.003, treatment 920, control 920, propensity score matching, day 28.
|
|
risk of death, 52.0% lower, HR 0.48, p = 0.048, treatment 1,490, control 1,372, propensity score weighting, day 14.
|
|
risk of death, 56.0% lower, HR 0.44, p = 0.003, treatment 920, control 920, propensity score matching, day 14.
|
|
risk of death, 87.0% lower, HR 0.13, p = 0.048, treatment 1,490, control 1,372, propensity score weighting, day 7.
|
|
risk of death, 88.0% lower, HR 0.12, p = 0.003, treatment 920, control 920, propensity score matching, day 7.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Zhong et al., 26 Dec 2024, retrospective, China, peer-reviewed, 7 authors, study period 1 December, 2022 - 31 March, 2023.
Contact: xianfa13579@163.com.
Azvudine efficacy in reducing mortality in COVID-19 patients
European Journal of Medical Research, doi:10.1186/s40001-024-02220-9
Background Several therapeutic drugs have been authorized for the treatment of patients with Coronavirus disease 2019 . However, further research on the mechanisms of action, efficacy, and target populations of these novel therapeutic drugs are necessary. This study included mild, moderate, severe, and critical COVID-19 patients to evaluate azvudine's effectiveness across different severity levels.
Methods We conducted a retrospective cohort study of patients with COVID-19 admitted to our hospital from December 1, 2022, to March 31, 2023. Patients were divided into retrospective cohorts receiving azvudine antiviral therapy and standard treatment, and were followed-up for up to 28 days.
Results Prior to data processing, azvudine treatment was associated with reduced mortality rates at 7 days (1.09/1000 persons vs. 5.06/1000 persons, P < 0.001) and 14 days (3.35/1000 persons vs. 5.65/1000 persons, P = 0.001). After propensity score matching, a decrease in mortality rates at 7 days (0.8/1000 persons vs. 6.29/1000 persons, P < 0.001), 14 days (3.42/1000 persons vs. 7.26/1000 persons, P < 0.001), and 28 days (4.33/1000 persons vs. 7.29/1000 persons, P = 0.003) were observed following azvudine treatment. After inverse probability of treatment weighting adjustment, the results were consistent with propensity score matching. In the clinical subgroup analysis, azvudine treatment intervention significantly reduced the 7-day (2.49/1000 persons vs. 14.59/1000 persons, P = 0.001 and 11.36/1000 persons vs. 66.99/1000 persons, P < 0.001), 14-day (5.22/1000 persons vs. 17.36/1000 persons, P < 0.001 and 17.08/1000 persons vs. 51.72/1000 persons, P = 0.002), and 28-day (7.58/1000 persons vs. 16.02/1000 persons, P = 0.014 and 20.43/1000 persons vs. 46.51/1000 persons, P = 0.008) mortality rates in hospitalized patients with severe and critical COVID-19.
Conclusions The study suggests that in hospitalized patients with COVID-19, azvudine treatment significantly reduces patient mortality rates in hospitalized COVID-19 infections, wherein the effects are more pronounced in severe and critical patients.
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi . org/ 10. 1186/ s40001-024-02220-9. Additional file 1
Author contributions XianfaL and JZ designed the experiments. XiaoL was responsible for clinical assessment of patients. LZ, XZ, LR, and ZZ collected the data. JZ was responsible for data management. JZ and ZZ conducted the statistical analysis. This article was written by ZZ, and reviewed by XianfaL. All the authors have reviewed and approved of the final manuscript.
Declarations Ethics approval and consent to participate There was no direct patient involvement in the conception, design, or implementation of this study. The requirement for patient consent was waived for
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Bernal, Molnupiravir for oral treatment of covid-19 in nonhospitalized patients, N Engl J Med, doi:10.1056/NEJMoa2116044
Bertuccio, The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: a retrospective study, Infection, doi:10.1007/s15010-023-02028-5
Chang, 4'-modified nucleosides for antiviral drug discovery: achievements and perspectives, Acc Chem Res, doi:10.1021/acs.accounts.1c00697
Da Silva, Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front Med, doi:10.3389/fmed.2023.1143485
Deng, Real-world effectiveness of Azvudine versus nirmatrelvirritonavir in hospitalized patients with COVID-19: a retrospective cohort study, J Med Virol, doi:10.1002/jmv.28756
Gentile, Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study, Vaccines, doi:10.3390/vaccines10101731
Hammond, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Hashemian, Paxlovid (Nirmatrelvir/Ritonavir): a new approach to Covid-19 therapy?, Biomed Pharmacother Biomedecine & Pharmacotherapie, doi:10.1016/j.biopha.2023.114367
Kabinger, Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis, Nat Struct Mol Biol, doi:10.1038/s41594-021-00651-0
Lui, Analysis of all-cause hospitalization and death among nonhospitalized patients with type 2 diabetes and SARS-CoV-2 infection treated with molnupiravir or nirmatrelvir-ritonavir during the omicron wave in Hong Kong, JAMA Netw Open, doi:10.1001/jamanetworkopen.2023.14393
Morris, Lee, Nirmatrelvir for nonhospitalized adults with Covid-19, N Engl J Med, doi:10.1056/NEJMc2206277
Ren, A Randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv Sci, doi:10.1002/advs.202001435
Sun, Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, EClinicalMedicine, doi:10.1016/j.eclinm.2023.101981
Wang, Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis, Virol J, doi:10.1186/s12985-024-02316-y
Wang, Efficacy and safety of azvudine for the treatment of COVID-19: a multicenter, open-label, randomized controlled trial, J Infect, doi:10.1016/j.jinf.2021.05.008
Wong, Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet, doi:10.1016/S0140-6736(22)01586-0
Xie, Molnupiravir and risk of post-acute sequelae of covid-19: cohort study, BMJ, doi:10.1136/bmj-2022-074572
Xie, Wang, Xu, Effectiveness and safety of azvudine versus nirmatrelvir-ritonavir in adult patients infected with COVID-19 omicron strains: a retrospective study in Beijing, Sci Rep, doi:10.1038/s41598-024-74502-5
Yang, Rao, Structural biology of SARS-CoV-2 and implications for therapeutic development, Nat Rev Microbiol, doi:10.1038/s41579-021-00630-8
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduction Targeted Therapy, doi:10.1038/s41392-020-00351-z
Zhang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther, doi:10.1038/s41392-021-00835-6
Zhao, Is azvudine comparable to nirmatrelvir-ritonavir in realworld efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study, Infect Dis Therapy, doi:10.1007/s40121-023-00845-7
Zhou, β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to Mammalian cells, J Infect Dis, doi:10.1093/infdis/jiab247
Zhu, Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials, Front Pharmacol, doi:10.3389/fphar.2023.1228548
DOI record:
{
"DOI": "10.1186/s40001-024-02220-9",
"ISSN": [
"2047-783X"
],
"URL": "http://dx.doi.org/10.1186/s40001-024-02220-9",
"alternative-id": [
"2220"
],
"article-number": "625",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "24 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "14 December 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "26 December 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "There was no direct patient involvement in the conception, design, or implementation of this study. The requirement for patient consent was waived for this retrospective study, which utilized data from electronic medical records. This study was approved by the Ethics Committee of the First Affiliated Hospital of the Gannan Medical University Hospital (LLSL-2024065)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Zhong",
"given": "Zhen",
"sequence": "first"
},
{
"affiliation": [],
"family": "Liu",
"given": "Xiao-feng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Xiao-zhong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhong",
"given": "Jia-ning",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Li-cheng",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Rong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Xian-fa",
"sequence": "additional"
}
],
"container-title": "European Journal of Medical Research",
"container-title-short": "Eur J Med Res",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
12,
26
]
],
"date-time": "2024-12-26T08:39:39Z",
"timestamp": 1735202379000
},
"deposited": {
"date-parts": [
[
2024,
12,
26
]
],
"date-time": "2024-12-26T09:05:31Z",
"timestamp": 1735203931000
},
"funder": [
{
"award": [
"202310802"
],
"name": "Health Commission of Jiangxi Province,China江西省卫生健康委员会"
},
{
"DOI": "10.13039/501100004479",
"award": [
"82060363"
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100004479",
"id-type": "DOI"
}
],
"name": "Natural Science Foundation of Jiangxi Province"
},
{
"award": [
"XN202023"
],
"name": "Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases,Ministry of Education"
}
],
"indexed": {
"date-parts": [
[
2024,
12,
27
]
],
"date-time": "2024-12-27T05:18:17Z",
"timestamp": 1735276697716,
"version": "3.32.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
12,
26
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
26
]
],
"date-time": "2024-12-26T00:00:00Z",
"timestamp": 1735171200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
12,
26
]
],
"date-time": "2024-12-26T00:00:00Z",
"timestamp": 1735171200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40001-024-02220-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40001-024-02220-9/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40001-024-02220-9.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
12,
26
]
]
},
"published-online": {
"date-parts": [
[
2024,
12,
26
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1056/NEJMc2206277",
"author": "AM Morris",
"doi-asserted-by": "publisher",
"first-page": "474",
"issue": "5",
"journal-title": "N Engl J Med",
"key": "2220_CR1",
"unstructured": "Morris AM, Lee TC. Nirmatrelvir for nonhospitalized adults with Covid-19. N Engl J Med. 2022;387(5):474–5. https://doi.org/10.1056/NEJMc2206277.",
"volume": "387",
"year": "2022"
},
{
"DOI": "10.3390/vaccines10101731",
"author": "I Gentile",
"doi-asserted-by": "publisher",
"first-page": "1731",
"issue": "10",
"journal-title": "Vaccines",
"key": "2220_CR2",
"unstructured": "Gentile I, et al. Nirmatrelvir/ritonavir and molnupiravir in the treatment of mild/moderate COVID-19: results of a real-life study. Vaccines. 2022;10(10):1731. https://doi.org/10.3390/vaccines10101731.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"issue": "10359",
"journal-title": "Lancet (London, England)",
"key": "2220_CR3",
"unstructured": "Wong CKH, et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet (London, England). 2022;400(10359):1213–22. https://doi.org/10.1016/S0140-6736(22)01586-0.",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1136/bmj-2022-074572",
"author": "Y Xie",
"doi-asserted-by": "publisher",
"first-page": "e074572",
"journal-title": "BMJ",
"key": "2220_CR4",
"unstructured": "Xie Y, et al. Molnupiravir and risk of post-acute sequelae of covid-19: cohort study. BMJ. 2023;381:e074572. https://doi.org/10.1136/bmj-2022-074572.",
"volume": "381",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "J-L Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "2220_CR5",
"unstructured": "Zhang J-L, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6(1):414. https://doi.org/10.1038/s41392-021-00835-6.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1007/s15010-023-02028-5",
"author": "P Bertuccio",
"doi-asserted-by": "publisher",
"first-page": "1633",
"issue": "6",
"journal-title": "Infection",
"key": "2220_CR6",
"unstructured": "Bertuccio P, et al. The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: a retrospective study. Infection. 2023;51(6):1633–44. https://doi.org/10.1007/s15010-023-02028-5.",
"volume": "51",
"year": "2023"
},
{
"DOI": "10.1001/jamanetworkopen.2023.14393",
"author": "DTW Lui",
"doi-asserted-by": "publisher",
"first-page": "e2314393",
"issue": "5",
"journal-title": "JAMA Netw Open",
"key": "2220_CR7",
"unstructured": "Lui DTW, et al. Analysis of all-cause hospitalization and death among nonhospitalized patients with type 2 diabetes and SARS-CoV-2 infection treated with molnupiravir or nirmatrelvir-ritonavir during the omicron wave in Hong Kong. JAMA Netw Open. 2023;6(5):e2314393. https://doi.org/10.1001/jamanetworkopen.2023.14393.",
"volume": "6",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "J Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"issue": "15",
"journal-title": "N Engl J Med",
"key": "2220_CR8",
"unstructured": "Hammond J, et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N Engl J Med. 2022;386(15):1397–408. https://doi.org/10.1056/NEJMoa2118542.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1016/j.biopha.2023.114367",
"author": "SMR Hashemian",
"doi-asserted-by": "publisher",
"first-page": "114367",
"journal-title": "Biomed Pharmacother Biomedecine & Pharmacotherapie",
"key": "2220_CR9",
"unstructured": "Hashemian SMR, et al. Paxlovid (Nirmatrelvir/Ritonavir): a new approach to Covid-19 therapy? Biomed Pharmacother Biomedecine & Pharmacotherapie. 2023;162:114367. https://doi.org/10.1016/j.biopha.2023.114367.",
"volume": "162",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2116044",
"author": "A Jayk Bernal",
"doi-asserted-by": "publisher",
"first-page": "509",
"issue": "6",
"journal-title": "N Engl J Med",
"key": "2220_CR10",
"unstructured": "Jayk Bernal A, et al. Molnupiravir for oral treatment of covid-19 in nonhospitalized patients. N Engl J Med. 2022;386(6):509–20. https://doi.org/10.1056/NEJMoa2116044.",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41594-021-00651-0",
"author": "F Kabinger",
"doi-asserted-by": "publisher",
"first-page": "740",
"issue": "9",
"journal-title": "Nat Struct Mol Biol",
"key": "2220_CR11",
"unstructured": "Kabinger F, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28(9):740–6. https://doi.org/10.1038/s41594-021-00651-0.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1093/infdis/jiab247",
"author": "S Zhou",
"doi-asserted-by": "publisher",
"first-page": "415",
"issue": "3",
"journal-title": "J Infect Dis",
"key": "2220_CR12",
"unstructured": "Zhou S, et al. β-d-N4-hydroxycytidine Inhibits SARS-CoV-2 through lethal mutagenesis but is also mutagenic to Mammalian cells. J Infect Dis. 2021;224(3):415–9. https://doi.org/10.1093/infdis/jiab247.",
"volume": "224",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"author": "B Yu",
"doi-asserted-by": "publisher",
"first-page": "236",
"issue": "1",
"journal-title": "Signal Transduction Targeted Therapy",
"key": "2220_CR13",
"unstructured": "Yu B, Chang J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduction Targeted Therapy. 2020;5(1):236. https://doi.org/10.1038/s41392-020-00351-z.",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1038/s41579-021-00630-8",
"author": "H Yang",
"doi-asserted-by": "publisher",
"first-page": "685",
"issue": "11",
"journal-title": "Nat Rev Microbiol",
"key": "2220_CR14",
"unstructured": "Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat Rev Microbiol. 2021;19(11):685–700. https://doi.org/10.1038/s41579-021-00630-8.",
"volume": "19",
"year": "2021"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Z Ren",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"issue": "19",
"journal-title": "Adv Sci",
"key": "2220_CR15",
"unstructured": "Ren Z, et al. A Randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci. 2020;7(19):e2001435. https://doi.org/10.1002/advs.202001435.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.3389/fphar.2023.1228548",
"author": "K-W Zhu",
"doi-asserted-by": "publisher",
"first-page": "24",
"issue": "1228548",
"journal-title": "Front Pharmacol",
"key": "2220_CR16",
"unstructured": "Zhu K-W. Efficacy and safety evaluation of Azvudine in the prospective treatment of COVID-19 based on four phase III clinical trials. Front Pharmacol. 2023;14(1228548):24. https://doi.org/10.3389/fphar.2023.1228548.",
"volume": "14",
"year": "2023"
},
{
"DOI": "10.1038/s41598-024-74502-5",
"author": "H Xie",
"doi-asserted-by": "publisher",
"first-page": "23974",
"journal-title": "Sci Rep",
"key": "2220_CR17",
"unstructured": "Xie H, Wang Y, Xu Y, et al. Effectiveness and safety of azvudine versus nirmatrelvir-ritonavir in adult patients infected with COVID-19 omicron strains: a retrospective study in Beijing. Sci Rep. 2024;14:23974. https://doi.org/10.1038/s41598-024-74502-5.",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.3760/cma.j.issn.1674-2397.2023.01.001",
"doi-asserted-by": "publisher",
"key": "2220_CR18",
"unstructured": "National Health Commission of the People’s Republic of China. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 10). Chin J Clin Infect Dis. 2023; 16(01): 1–9. https://doi.org/10.3760/cma.j.issn.1674-2397.2023.01.001."
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"author": "Y Sun",
"doi-asserted-by": "publisher",
"first-page": "101981",
"journal-title": "EClinicalMedicine",
"key": "2220_CR19",
"unstructured": "Sun Y, et al. Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. EClinicalMedicine. 2023;59:101981. https://doi.org/10.1016/j.eclinm.2023.101981.",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1007/s40121-023-00845-7",
"author": "Q Zhao",
"doi-asserted-by": "publisher",
"first-page": "2087",
"issue": "8",
"journal-title": "Infect Dis Therapy",
"key": "2220_CR20",
"unstructured": "Zhao Q, et al. Is azvudine comparable to nirmatrelvir-ritonavir in real-world efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study. Infect Dis Therapy. 2023;12(8):2087–102. https://doi.org/10.1007/s40121-023-00845-7.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3389/fmed.2023.1143485",
"author": "RM da Silva",
"doi-asserted-by": "publisher",
"first-page": "1143485",
"journal-title": "Front Med",
"key": "2220_CR21",
"unstructured": "da Silva RM, et al. Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front Med. 2023;10:1143485. https://doi.org/10.3389/fmed.2023.1143485.",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28756",
"author": "G Deng",
"doi-asserted-by": "publisher",
"first-page": "e28756",
"issue": "4",
"journal-title": "J Med Virol",
"key": "2220_CR22",
"unstructured": "Deng G, et al. Real-world effectiveness of Azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J Med Virol. 2023;95(4):e28756. https://doi.org/10.1002/jmv.28756.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1186/s12985-024-02316-y",
"author": "Y Wang",
"doi-asserted-by": "publisher",
"first-page": "46",
"issue": "1",
"journal-title": "Virol J",
"key": "2220_CR23",
"unstructured": "Wang Y, et al. Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis. Virol J. 2024;21(1):46. https://doi.org/10.1186/s12985-024-02316-y.",
"volume": "21",
"year": "2024"
},
{
"DOI": "10.1021/acs.accounts.1c00697",
"author": "J Chang",
"doi-asserted-by": "publisher",
"first-page": "565",
"issue": "4",
"journal-title": "Acc Chem Res",
"key": "2220_CR24",
"unstructured": "Chang J. 4’-modified nucleosides for antiviral drug discovery: achievements and perspectives. Acc Chem Res. 2022;55(4):565–78. https://doi.org/10.1021/acs.accounts.1c00697.",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1016/j.jinf.2021.05.008",
"doi-asserted-by": "publisher",
"key": "2220_CR25",
"unstructured": "Wang, Yaqi, et al. Efficacy and safety of azvudine for the treatment of COVID-19: a multicenter, open-label, randomized controlled trial. J Infect. 2021;83(3): 346–355. https://doi.org/10.1016/j.jinf.2021.05.008."
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://eurjmedres.biomedcentral.com/articles/10.1186/s40001-024-02220-9"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Azvudine efficacy in reducing mortality in COVID-19 patients",
"type": "journal-article",
"update-policy": "https://doi.org/10.1007/springer_crossmark_policy",
"volume": "29"
}
