Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis
et al., Virology Journal, doi:10.1186/s12985-024-02316-y, Feb 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
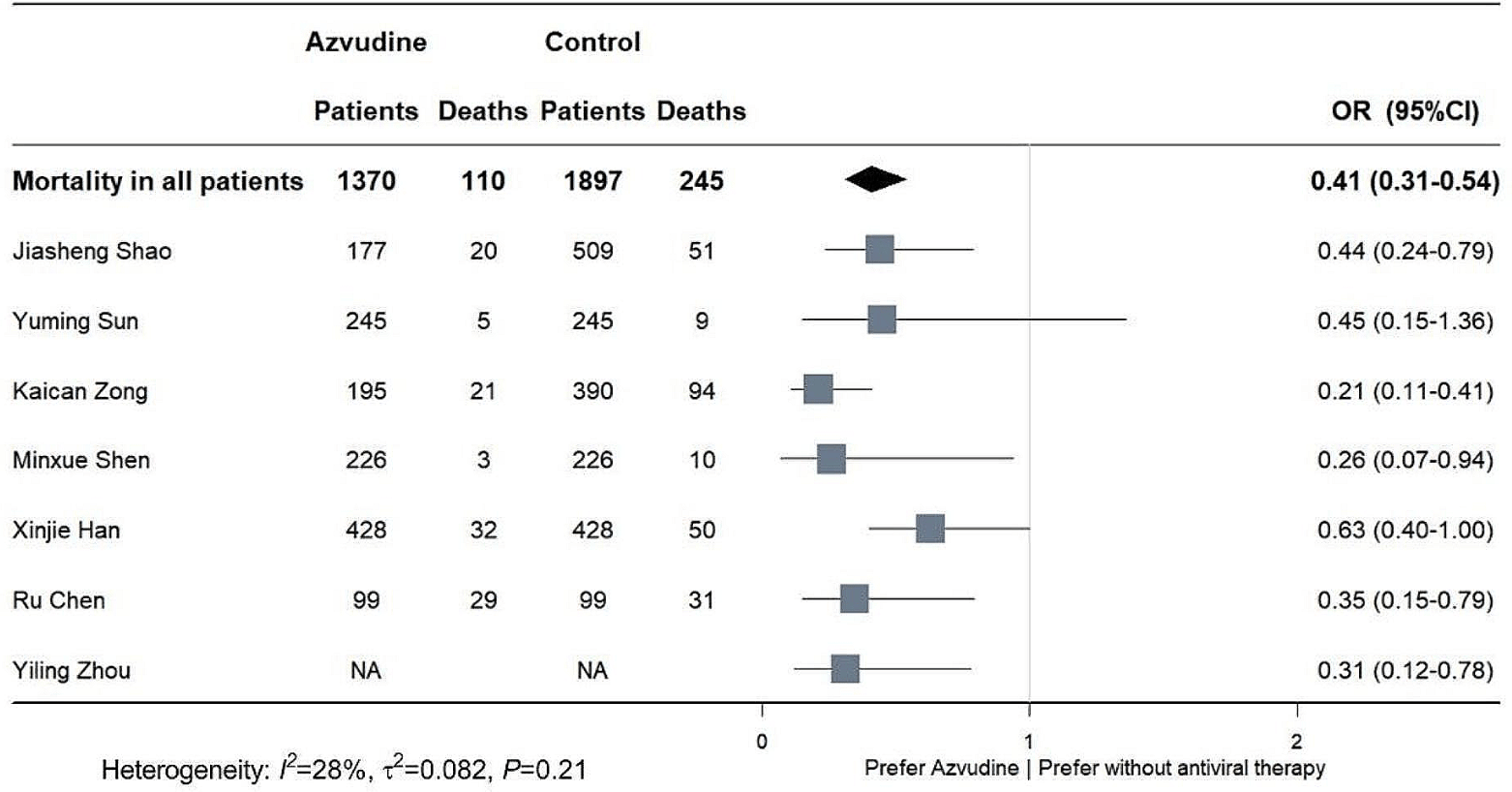
Systematic review and meta-analysis of 17 studies showing significantly lower mortality with azvudine compared to no antiviral treatment in COVID-19 patients. The mortality benefit was seen in both mild/moderate and severe disease, as well as in patients over 65 years old. There was no significant difference found between azvudine and control groups for ICU admission, invasive ventilation, or adverse events.
5 meta-analyses show significant improvements with azvudine for mortality3-7,
mechanical ventilation3,
improvement3,
progression7, and
viral clearance3,5,6 .
Currently there are 39 azvudine for COVID-19 studies, showing 30% lower mortality [20‑39%], 18% lower ventilation [-10‑39%], 21% lower ICU admission [5‑34%], and 10% lower hospitalization [0‑19%].
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
2.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
3.
Zheng et al., Small-molecule antivirals treatment for COVID-19: A systematic review and network meta-analysis, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2024.107096.
4.
Wang (B) et al., Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis, Virology Journal, doi:10.1186/s12985-024-02316-y.
5.
Amani et al., Effectiveness and safety of azvudine in COVID-19: A systematic review and meta-analysis, PLOS ONE, doi:10.1371/journal.pone.0298772.
Wang et al., 23 Feb 2024, peer-reviewed, 13 authors.
Contact: tianxl@pumch.cn.
Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis
Virology Journal, doi:10.1186/s12985-024-02316-y
Background Azvudine has been approved for the treatment of coronavirus disease 2019 patients in China, and this meta-analysis aims to illustrate the safety of azvudine and its effectiveness in reducing mortality. Methods PubMed, Embase, Web of science, Cochrane Library and the Epistemonikos COVID-19 Living Overview of Evidence database (L.OVE) were searched to aggregate currently published studies. Cochrane risk of bias tool and ROBINS-I tool were used to assess the risk of bias of randomized controlled study and cohort study respectively. Odds radios (ORs) with 95% confidence interval (CIs) were combined for dichotomous variables. Publication bias was assessed by Egger's test and funnel plots. Results A total of 184 articles were retrieved from the included databases and 17 studies were included into the final analysis. Pooled analysis showed that azvudine significantly reduced mortality risk in COVID-19 patients compared with controls (OR: 0.41, 95%CI 0.31-0.54, p < 0.001). Besides, either mild to moderate or severe COVID-19 patients could benefit from azvudine administration. There was no significant difference in the incidence of ICU admission (OR: 0.90, 95%CI 0.47-1.72, p = 0.74) and invasive ventilation (OR: 0.94, 95%CI 0.54-1.62, p = 0.82) between azvudine and control group. The incidence of adverse events was similar between azvudine and control (OR: 1.26, 95%CI 0.59-2.70, p = 0.56).
Conclusions This meta-analysis suggests that azvudine could reduce the mortality risk of COVID-19 patients, and the safety of administration is acceptable.
Abbreviations
Supplementary Information The online version contains supplementary material available at https://doi. org/10.1186/s12985-024-02316-y.
Supplementary Material 1: Supplementary
Declarations Ethics approval and consent to participate Not applicable.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Amani, Amani, Efficacy and safety of nirmatrelvir/ritonavir (paxlovid) for COVID-19: a rapid review and meta-analysis, J Med Virol
Cabral, De Souza, Silva, Arruda, Cabral et al., Serial viral load analysis by Ddpcr to evaluate Fnc efficacy and safety in the treatment of moderate cases of Covid-19
Cheema, Jafar, Sohail, Shahid, Sahra et al., Nirmatrelvir-Ritonavir for the treatment of COVID-19 patients: a systematic review and meta-analysis, J Med Virol
Chen, Guo, Deng, Wang, Gao et al., Allcause mortality in moderate and severe COVID-19 patients with myocardial injury receiving versus not receiving azvudine: a propensity score-matched analysis, Cardiol Plus
Chen, Tian, Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis, Heliyon
Chen, Xu, Hong, Yang, Peng et al., Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 Omicron variant. A retrospective cohort study
Chu, Schwartz, Donnelly, Chuey, Soto et al., Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection, JAMA Intern Med
Da Silva, Cabral, De Souza, Arruda, Cabral et al., Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front Med
Deng, Li, Sun, Zhou, Xiao et al., Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study, J Med Virol
Dian, Meng, Sun, Deng, Zeng, Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities, J Infect
Docherty, Harrison, Green, Hardwick, Pius et al., Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study, BMJ
Gao, Luo, Ren, Duan, Han et al., Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19, J Infect
Han, Han, Wang, Wang, Cui et al., Effectiveness and optimal timing of azvudine in COVID-19 patients: a multicenter retrospective study in Beijing
Higgins, Altman, Gøtzsche, Jüni, Moher et al., The Cochrane collaboration's tool for assessing risk of bias in randomised trials, BMJ
Keni, Alexander, Nayak, Mudgal, Nandakumar, COVID-19: emergence, spread, possible treatments, and global burden, Front Public Health
Lamontagne, Agarwal, Rochwerg, Siemieniuk, Agoritsas et al., A living WHO guideline on drugs for covid-19, BMJ
Lemaitre, Grégoire, Monchaud, Bouchet, Saint-Salvi et al., Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for Covid-19: guidelines from the French society of pharmacology and therapeutics (SFPT), Therapie
Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche et al., The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration, BMJ
Ren, Luo, Yu, Song, Liang et al., A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study, Adv Sci
Shang, Fu, Geng, Zhang, Zhang et al., Azvudine therapy of common COVID-19 in hemodialysis patients, J Med Virol
Shao, Fan, Guo, Huang, Guo et al., Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China, Microorganisms
Shen, Xiao, Sun, Li, Wu et al., Real-world effectiveness of azvudine in hospitalized patients with COVID-19: a retrospective cohort study, medRxiv
Sterne, Hernán, Reeves, Savović, Berkman et al., ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions, BMJ
Sun, Dian, Shen, Zeng, Chen et al., Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, Eclinicalmedicine
Sun, Peng, Yu, Zhang, Liang et al., Mechanistic insight into antiretroviral potency of 2'-Deoxy-2'-β-fluoro-4'-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention, J Med Chem
Tian, Sun, Xu, Ye, The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant, J Med Virol
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong's omicron BA.2 wave: a retrospective cohort study, Lancet Infect Dis
Wong, Au, Lau, Lau, Cowling et al., Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study, Lancet
Yang, Wang, Jiang, Zhang, Zhang et al., Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study, J Med Virol
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduct Target Therapy
Zhang, Li, Wang, Liu, Lu et al., Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther
Zhao, Zheng, Han, Feng, Xia et al., Is azvudine comparable to nirmatrelvir-ritonavir in real-world efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study, Infect Dis Ther
Zhou, Liu, Jiang, Zhang, Zhang et al., -to-severe covid-19: emulation of a randomised target trial
Zong, Zhou, Li, Jiang, Liu et al., Azvudine reduces the in-hospital mortality of COVID-19 patients: a retrospective cohort study, Acta Pharm Sinica B
DOI record:
{
"DOI": "10.1186/s12985-024-02316-y",
"ISSN": [
"1743-422X"
],
"URL": "http://dx.doi.org/10.1186/s12985-024-02316-y",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Azvudine has been approved for the treatment of coronavirus disease 2019 (COVID-19) patients in China, and this meta-analysis aims to illustrate the safety of azvudine and its effectiveness in reducing mortality.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>PubMed, Embase, Web of science, Cochrane Library and the Epistemonikos COVID-19 Living Overview of Evidence database (L.OVE) were searched to aggregate currently published studies. Cochrane risk of bias tool and ROBINS-I tool were used to assess the risk of bias of randomized controlled study and cohort study respectively. Odds radios (ORs) with 95% confidence interval (CIs) were combined for dichotomous variables. Publication bias was assessed by Egger’s test and funnel plots.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>A total of 184 articles were retrieved from the included databases and 17 studies were included into the final analysis. Pooled analysis showed that azvudine significantly reduced mortality risk in COVID-19 patients compared with controls (OR: 0.41, 95%CI 0.31–0.54, <jats:italic>p</jats:italic> < 0.001). Besides, either mild to moderate or severe COVID-19 patients could benefit from azvudine administration. There was no significant difference in the incidence of ICU admission (OR: 0.90, 95%CI 0.47–1.72, <jats:italic>p</jats:italic> = 0.74) and invasive ventilation (OR: 0.94, 95%CI 0.54–1.62, <jats:italic>p</jats:italic> = 0.82) between azvudine and control group. The incidence of adverse events was similar between azvudine and control (OR: 1.26, 95%CI 0.59–2.70, <jats:italic>p</jats:italic> = 0.56).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>This meta-analysis suggests that azvudine could reduce the mortality risk of COVID-19 patients, and the safety of administration is acceptable.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>PROSPERO; No.: CRD42023462988; URL: <jats:ext-link xmlns:xlink=\"http://www.w3.org/1999/xlink\" ext-link-type=\"uri\" xlink:href=\"https://www.crd.york.ac.uk/prospero/\">https://www.crd.york.ac.uk/prospero/</jats:ext-link>.</jats:p>\n </jats:sec>",
"alternative-id": [
"2316"
],
"article-number": "46",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "27 November 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 February 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "23 February 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Not applicable."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Wang",
"given": "Yaqi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Xie",
"given": "Huaiya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Luo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fan",
"given": "Junping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Ying",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pan",
"given": "Siqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhou",
"given": "Wangji",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Qiaoling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liu",
"given": "Xueqi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Aohua",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Hong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Jinglan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tian",
"given": "Xinlun",
"sequence": "additional"
}
],
"container-title": "Virology Journal",
"container-title-short": "Virol J",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T19:02:30Z",
"timestamp": 1708714950000
},
"deposited": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T19:03:00Z",
"timestamp": 1708714980000
},
"funder": [
{
"award": [
"2023YFC3041900"
],
"name": "National Key Research and Development Program of China"
},
{
"award": [
"2021-I2M-1-048"
],
"name": "Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
24
]
],
"date-time": "2024-02-24T00:28:18Z",
"timestamp": 1708734498944
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
2,
23
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T00:00:00Z",
"timestamp": 1708646400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
2,
23
]
],
"date-time": "2024-02-23T00:00:00Z",
"timestamp": 1708646400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12985-024-02316-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12985-024-02316-y/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12985-024-02316-y.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
2,
23
]
]
},
"published-online": {
"date-parts": [
[
2024,
2,
23
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "2316_CR1",
"unstructured": "WHO COVID-19 Dashboard. Geneva: World Health Organization; 2020. Available online: https://covid19.who.int/"
},
{
"DOI": "10.3389/fpubh.2020.00216",
"author": "R Keni",
"doi-asserted-by": "publisher",
"first-page": "216",
"journal-title": "Front Public Health",
"key": "2316_CR2",
"unstructured": "Keni R, Alexander A, Nayak PG, Mudgal J, Nandakumar K. COVID-19: emergence, spread, possible treatments, and global burden. Front Public Health. 2020;8:216.",
"volume": "8",
"year": "2020"
},
{
"key": "2316_CR3",
"unstructured": "Joint prevention and control Mechnism of The State Council for the Novel Coronavirus Pneumonia. Notice on further optimizing and implementing the prevention and control measures of the novel coronavirus. [http://www.gov.cn/xinwen/2022-12/07/content_5730443.htm]"
},
{
"DOI": "10.1002/jmv.27643",
"author": "D Tian",
"doi-asserted-by": "publisher",
"first-page": "2376",
"journal-title": "J Med Virol",
"key": "2316_CR4",
"unstructured": "Tian D, Sun Y, Xu H, Ye Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J Med Virol. 2022;94:2376–83.",
"volume": "94",
"year": "2022"
},
{
"author": "F Lamontagne",
"first-page": "m3379",
"journal-title": "BMJ",
"key": "2316_CR5",
"unstructured": "Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, Lytvyn L, Leo YS, Macdonald H, Zeng L, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379.",
"volume": "370",
"year": "2020"
},
{
"key": "2316_CR6",
"unstructured": "General Office of the National Health Commission. Notice on including azovudine tablets into the diagnosis and treatment protocol for COVID-19 in China. [https://www.gov.cn/zhengce/zhengceku/2022-08/10/content_5704788.htm]"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "JL Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "Signal Transduct Target Ther",
"key": "2316_CR7",
"unstructured": "Zhang JL, Li YH, Wang LL, Liu HQ, Lu SY, Liu Y, Li K, Liu B, Li SY, Shao FM, et al. Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients. Signal Transduct Target Ther. 2021;6:414.",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"doi-asserted-by": "crossref",
"key": "2316_CR8",
"unstructured": "Yu B, Chang J. Azvudine (FNC): a promising clinical candidate for COVID-19 treatment. Signal Transduct Target Therapy. 2020;5."
},
{
"DOI": "10.1002/advs.202001435",
"doi-asserted-by": "crossref",
"key": "2316_CR9",
"unstructured": "Ren Z, Luo H, Yu Z, Song J, Liang L, Wang L, Wang H, Cui G, Liu Y, Wang J et al. A randomized, open-label, controlled clinical trial of azvudine tablets in the treatment of mild and common COVID-19, a pilot study. Adv Sci. 2020;7."
},
{
"DOI": "10.3389/fmed.2023.1143485",
"doi-asserted-by": "crossref",
"key": "2316_CR10",
"unstructured": "da Silva RM, Cabral PGA, de Souza SB, Arruda RF, Cabral SPF, de Assis ALEM, Martins YPM, Tavares CAdA, Viana Junior AB, Chang J et al. Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19. Front Med. 2023;10."
},
{
"DOI": "10.21203/rs.3.rs-2273657/v1",
"doi-asserted-by": "crossref",
"key": "2316_CR11",
"unstructured": "Cabral P, de Souza S, Silva R, Arruda R, Cabral S, de Assis A, Júnior A, Degrave W, Moreira A, Silva C et al. Serial viral load analysis by Ddpcr to evaluate Fnc efficacy and safety in the treatment of moderate cases of Covid-19. ResearchSquare. 2022."
},
{
"DOI": "10.2139/ssrn.4380058",
"doi-asserted-by": "crossref",
"key": "2316_CR12",
"unstructured": "Zong K, Zhou H, Li W, Jiang E, Liu Y, Li S. Azvudine reduces the in-hospital mortality of COVID-19 patients: a retrospective cohort study. Acta Pharm Sinica B. 2023."
},
{
"DOI": "10.1101/2023.01.23.23284899",
"doi-asserted-by": "crossref",
"key": "2316_CR13",
"unstructured": "Shen M, Xiao C, Sun Y, Li D, Wu P, Jin L, Wu Q, Dian Y, Meng Y, Zeng F et al. Real-world effectiveness of azvudine in hospitalized patients with COVID-19: a retrospective cohort study. medRxiv. 2023."
},
{
"DOI": "10.1002/jmv.28756",
"doi-asserted-by": "crossref",
"key": "2316_CR14",
"unstructured": "Deng G, Li D, Sun Y, Jin L, Zhou Q, Xiao C, Wu Q, Sun H, Dian Y, Zeng F et al. Real-world effectiveness of azvudine versus nirmatrelvir-ritonavir in hospitalized patients with COVID-19: a retrospective cohort study. J Med Virol. 2023;95."
},
{
"DOI": "10.1007/s40121-023-00845-7",
"doi-asserted-by": "crossref",
"key": "2316_CR15",
"unstructured": "Zhao Q, Zheng B, Han B, Feng P, Xia Z, Jiang H, Ying Y, Zhu J, Fei C, Xiang J, et al. Is azvudine comparable to nirmatrelvir-ritonavir in real-world efficacy and safety for hospitalized patients with COVID-19? A retrospective cohort study. Infect Dis Ther. 2023."
},
{
"DOI": "10.1016/j.heliyon.2023.e20153",
"author": "Z Chen",
"doi-asserted-by": "publisher",
"first-page": "e20153",
"journal-title": "Heliyon",
"key": "2316_CR16",
"unstructured": "Chen Z, Tian F. Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis. Heliyon. 2023;9:e20153.",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1136/bmj.b2700",
"author": "A Liberati",
"doi-asserted-by": "publisher",
"first-page": "b2700",
"journal-title": "BMJ",
"key": "2316_CR17",
"unstructured": "Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.",
"volume": "339",
"year": "2009"
},
{
"DOI": "10.1136/bmj.d5928",
"author": "JP Higgins",
"doi-asserted-by": "publisher",
"first-page": "d5928",
"journal-title": "BMJ",
"key": "2316_CR18",
"unstructured": "Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.",
"volume": "343",
"year": "2011"
},
{
"DOI": "10.1136/bmj.i4919",
"author": "JA Sterne",
"doi-asserted-by": "publisher",
"first-page": "i4919",
"journal-title": "BMJ",
"key": "2316_CR19",
"unstructured": "Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.",
"volume": "355",
"year": "2016"
},
{
"DOI": "10.1002/jmv.28947",
"author": "H Yang",
"doi-asserted-by": "publisher",
"first-page": "e28947",
"journal-title": "J Med Virol",
"key": "2316_CR20",
"unstructured": "Yang H, Wang Z, Jiang C, Zhang Y, Zhang Y, Xu M, Zhang Y, Wang Y, Liu X, An Z, et al. Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study. J Med Virol. 2023;95:e28947.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1101/2023.05.10.23289325",
"doi-asserted-by": "crossref",
"key": "2316_CR21",
"unstructured": "Shao J, Fan R, Guo C, Huang X, Guo R, Zhang F, Hu J, Huang G, Cao L. Composite interventions on outcomes of severely and critically ill patients with COVID-19 in Shanghai, China. Microorganisms. 2023;11."
},
{
"DOI": "10.1097/CP9.0000000000000049",
"author": "R Chen",
"doi-asserted-by": "publisher",
"first-page": "103",
"journal-title": "Cardiol Plus",
"key": "2316_CR22",
"unstructured": "Chen R, Guo Y, Deng S, Wang J, Gao M, Han H, Wang L, Jiang H, Huang K. All-cause mortality in moderate and severe COVID-19 patients with myocardial injury receiving versus not receiving azvudine: a propensity score-matched analysis. Cardiol Plus. 2023;8:103–10.",
"volume": "8",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29007",
"author": "S Shang",
"doi-asserted-by": "publisher",
"first-page": "e29007",
"journal-title": "J Med Virol",
"key": "2316_CR23",
"unstructured": "Shang S, Fu B, Geng Y, Zhang J, Zhang D, Xiao F, Sheng Z, Zhai J, Li W, Chen X, et al. Azvudine therapy of common COVID-19 in hemodialysis patients. J Med Virol. 2023;95:e29007.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1101/2023.01.05.23284180",
"doi-asserted-by": "crossref",
"key": "2316_CR24",
"unstructured": "Chen W, Xu H, Hong L, Yang R, Peng C, Wang G, Li W. Oral azvudine (FNC) tablets in patients infected with SARS-CoV-2 Omicron variant. A retrospective cohort study. 2023."
},
{
"DOI": "10.21203/rs.3.rs-3145554/v1",
"doi-asserted-by": "crossref",
"key": "2316_CR25",
"unstructured": "Han X, Han X, Wang Y, Wang Z, Cui J, Zhao W, Mo G, Liu Y, Zheng M, Xie F et al. Effectiveness and optimal timing of azvudine in COVID-19 patients: a multi-center retrospective study in Beijing, China. ResearchSquare. 2023."
},
{
"DOI": "10.1016/j.jinf.2023.05.012",
"author": "Y Dian",
"doi-asserted-by": "publisher",
"first-page": "E24",
"journal-title": "J Infect",
"key": "2316_CR26",
"unstructured": "Dian Y, Meng Y, Sun Y, Deng G, Zeng F. Azvudine versus paxlovid for oral treatment of COVID-19 in Chinese patients with pre-existing comorbidities. J Infect. 2023;87:E24–7.",
"volume": "87",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"author": "Y Gao",
"doi-asserted-by": "publisher",
"first-page": "E158",
"journal-title": "J Infect",
"key": "2316_CR27",
"unstructured": "Gao Y, Luo Z, Ren S, Duan Z, Han Y, Liu H, Gao Z, Zhang X, Hu Z, Ma Y. Antiviral effect of azvudine and nirmatrelvir-ritonavir among hospitalized patients with COVID-19. J Infect. 2023;86:E158–60.",
"volume": "86",
"year": "2023"
},
{
"DOI": "10.2139/ssrn.4478199",
"doi-asserted-by": "crossref",
"key": "2316_CR28",
"unstructured": "Zhou Y, Liu Y, Jiang L, Zhang R, Zhang H, Shi Q, Yang Z, Mao Y, Liu S, Yang Z, et al. Azvudine and nirmatrelvir–ritonavir in hospitalised patients with moderate–to–severe covid–19: emulation of a randomised target trial. SSRN; 2023."
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"doi-asserted-by": "crossref",
"key": "2316_CR29",
"unstructured": "Sun Y, Jin L, Dian Y, Shen M, Zeng F, Chen X, Deng G. Oral azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study. Eclinicalmedicine. 2023;59."
},
{
"DOI": "10.1016/S0140-6736(22)01586-0",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1213",
"journal-title": "Lancet",
"key": "2316_CR30",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet. 2022;400:1213–22.",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(22)00507-2",
"author": "CKH Wong",
"doi-asserted-by": "publisher",
"first-page": "1681",
"journal-title": "Lancet Infect Dis",
"key": "2316_CR31",
"unstructured": "Wong CKH, Au ICH, Lau KTK, Lau EHY, Cowling BJ, Leung GM. Real-world effectiveness of early molnupiravir or nirmatrelvir-ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s omicron BA.2 wave: a retrospective cohort study. Lancet Infect Dis. 2022;22:1681–93.",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28441",
"author": "B Amani",
"doi-asserted-by": "publisher",
"first-page": "e28441",
"journal-title": "J Med Virol",
"key": "2316_CR32",
"unstructured": "Amani B, Amani B. Efficacy and safety of nirmatrelvir/ritonavir (paxlovid) for COVID-19: a rapid review and meta-analysis. J Med Virol. 2023;95:e28441.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28471",
"author": "HA Cheema",
"doi-asserted-by": "publisher",
"first-page": "e28471",
"journal-title": "J Med Virol",
"key": "2316_CR33",
"unstructured": "Cheema HA, Jafar U, Sohail A, Shahid A, Sahra S, Ehsan M, Athar F, Shah J, Sah R. Nirmatrelvir-Ritonavir for the treatment of COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2023;95:e28471.",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1136/bmj.m1985",
"doi-asserted-by": "crossref",
"key": "2316_CR34",
"unstructured": "Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369m1985."
},
{
"DOI": "10.1001/jamainternmed.2022.1827",
"author": "VT Chu",
"doi-asserted-by": "publisher",
"first-page": "701",
"journal-title": "JAMA Intern Med",
"key": "2316_CR35",
"unstructured": "Chu VT, Schwartz NG, Donnelly MAP, Chuey MR, Soto R, Yousaf AR, Schmitt-Matzen EN, Sleweon S, Ruffin J, Thornburg N, et al. Comparison of home antigen testing with RT-PCR and viral culture during the course of SARS-CoV-2 infection. JAMA Intern Med. 2022;182:701–9.",
"volume": "182",
"year": "2022"
},
{
"DOI": "10.1016/j.therap.2022.03.005",
"author": "F Lemaitre",
"doi-asserted-by": "publisher",
"first-page": "509",
"journal-title": "Therapie",
"key": "2316_CR36",
"unstructured": "Lemaitre F, Grégoire M, Monchaud C, Bouchet S, Saint-Salvi B, Polard E. Management of drug-drug interactions with nirmatrelvir/ritonavir in patients treated for Covid-19: guidelines from the French society of pharmacology and therapeutics (SFPT). Therapie. 2022;77:509–21.",
"volume": "77",
"year": "2022"
},
{
"DOI": "10.1021/acs.jmedchem.0c00940",
"author": "L Sun",
"doi-asserted-by": "publisher",
"first-page": "8554",
"journal-title": "J Med Chem",
"key": "2316_CR37",
"unstructured": "Sun L, Peng Y, Yu W, Zhang Y, Liang L, Song C, Hou J, Qiao Y, Wang Q, Chen J, et al. Mechanistic insight into antiretroviral potency of 2’-Deoxy-2’-β-fluoro-4’-azidocytidine (FNC) with a long-lasting effect on HIV-1 prevention. J Med Chem. 2020;63:8554–66.",
"volume": "63",
"year": "2020"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://virologyj.biomedcentral.com/articles/10.1186/s12985-024-02316-y"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "21"
}
