Efficacy and Safety of Azvudine in Patients With COVID‐19 in China: A Meta‐Analysis of Observational Studies
et al., The Clinical Respiratory Journal, doi:10.1111/crj.13798, PROSPERO CRD42024520565, Jul 2024
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.000000017 from 39 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
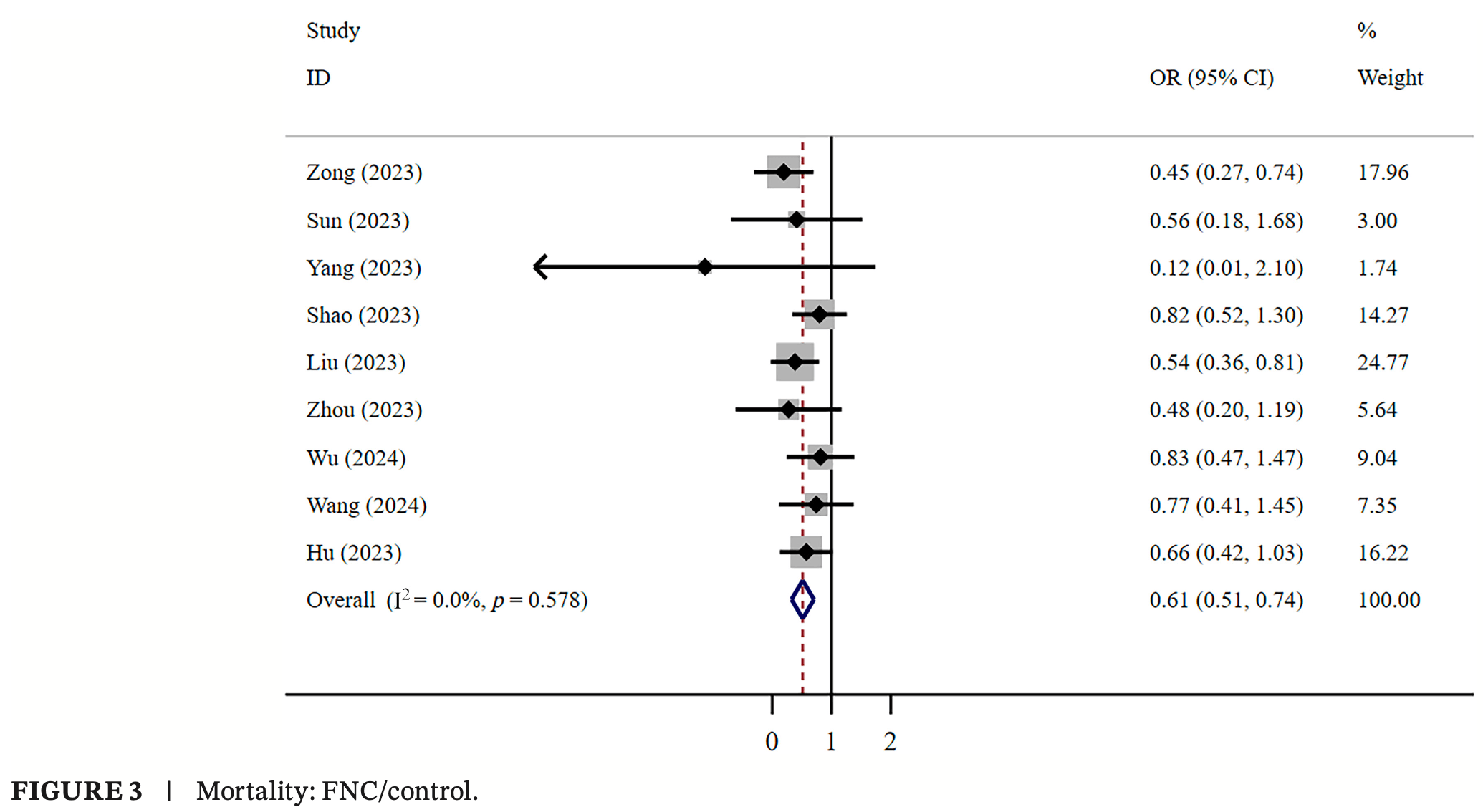
Meta analysis of 24 studies with 11,830 COVID-19 patients in China, showing significantly lower mortality, lower combined mortality/ventilation/ICU admission, and faster viral clearance with azvudine compared to SOC. In studies comparing with paxlovid, there were fewer adverse events with azvudine, but a longer time to first nucleic acid negative conversion.
5 meta-analyses show significant improvements with azvudine for mortality1-5,
mechanical ventilation1,
improvement1,
progression5, and
viral clearance1,3,4 .
Currently there are 39 azvudine for COVID-19 studies, showing 30% lower mortality [20‑39%], 18% lower ventilation [-10‑39%], 21% lower ICU admission [5‑34%], and 10% lower hospitalization [0‑19%].
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments6.
|
risk of death, 39.0% lower, OR 0.61, p < 0.001, RR approximated with OR.
|
|
death/mechanical ventilation/ICU, 33.0% lower, OR 0.67, p = 0.009, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zheng et al., Small-molecule antivirals treatment for COVID-19: A systematic review and network meta-analysis, International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2024.107096.
2.
Wang et al., Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis, Virology Journal, doi:10.1186/s12985-024-02316-y.
3.
Amani et al., Effectiveness and safety of azvudine in COVID-19: A systematic review and meta-analysis, PLOS ONE, doi:10.1371/journal.pone.0298772.
4.
Dong et al., Efficacy and Safety of Azvudine in Patients With COVID‐19 in China: A Meta‐Analysis of Observational Studies, The Clinical Respiratory Journal, doi:10.1111/crj.13798.
Dong et al., 12 Jul 2024, China, peer-reviewed, 7 authors, trial PROSPERO CRD42024520565.
Contact: yunaliu@126.com.
Efficacy and Safety of Azvudine in Patients With COVID‐19 in China: A Meta‐Analysis of Observational Studies
The Clinical Respiratory Journal, doi:10.1111/crj.13798
Background: Azvudine (FNC) is a novel small molecule antiviral drug for treating COVID-19 that is available only on the Chinese market. Despite being recommended for treating COVID-19 by the Chinese guidelines, its efficacy and safety are still unclear. This study aimed to evaluate the protective effect of FNC on COVID-19 outcomes and its safety. Methods: We followed the PRISMA 2020 guidelines and searched the PubMed, Embase, Web of Science, Scopus, and China National Knowledge Infrastructure (CNKI) databases to evaluate studies on the effectiveness of FNC in treating COVID-19 in China, focusing on mortality and overall outcomes. Additionally, its impact on the length of hospital stay (LOHS), time to first nucleic acid negative conversion (T-FNANC), and adverse events was evaluated. The inclusion criterion was that the studies were published from July 2021 to April 10, 2024. This study uses the ROBINS-I tool to assess bias risk and employs the GRADE approach to evaluate the certainty of the evidence.
Results: The meta-analysis included 24 retrospective studies involving a total of 11 830 patients. Low-certainty evidence revealed no significant difference in mortality (OR = 0.91, 95% CI: 0.76-1.08) or LOHS (WMD = -0.24, 95% CI: -0.83 to 0.35) between FNC and Paxlovid in COVID-19 patients. Low-certainty evidence shows that the T-FNANC was longer (WMD = 1.95, 95% CI: 0.36-3.53). Compared with the Paxlovid group, low-certainty evidence shows the FNC group exhibited a worse composite outcome (OR = 0.77, 95% CI: 0.63-0.95) and fewer adverse events (OR = 0.63, 95% CI: 0.46-0.85). Compared with supportive treatment, low certainty shows FNC significantly reduced the mortality rate in COVID-19 patients (OR = 0.61, 95% CI: 0.51-0.74) and decreased the composite outcome (OR = 0.67, 95% CI: 0.50-0.91), and very low certainty evidence shows significantly decreased the T-FNANC (WMD = -4.62, 95% CI: -8.08 to -1.15). However, in very low certainty, there was no significant difference in LOHS (WMD = -0.70, 95% CI: -3.32 to 1.91) or adverse events (OR = 1.97, 95% CI: 0.48-8.17). Conclusions: FNC appears to be a safe and potentially effective treatment for COVID-19 in China, but further research with larger, high-quality studies is necessary to confirm these findings. Due to the certainty of the evidence and the specific context of the studies conducted in China, caution should be exercised when considering whether the results are applicable worldwide.
Author Contributions Study design: Wentao Zhang, Tao Dong, and Yuna Liu. Data collection: Wentao Zhang, Tingting Wu, Tao Dong, Yongxiang Ge, Qi Yang, and Jia Xu. Data analysis: Wentao Zhang and Tao Dong. Writing: Wentao Zhang, Tingting Wu, and Yuna Liu. All authors have reviewed the manuscript.
Ethics Statement The authors have nothing to report.
Conflicts of Interest The authors declare no conflicts of interest.
Supporting Information Additional supporting information can be found online in the Supporting Information section.
References
Chen, Tian, Efficacy and Safety of Azvudine in Patients With COVID-19: A Systematic Review and Meta-Analysis, Heliyon
Chen, Xu, Hong, Oral Azvudine (FNC) Tablets in Patients Infected With SARS-CoV-2 Omicron Variant: A Retrospective Cohort Study
Deng, Li, Sun, Real-World Effectiveness of Azvudine Versus Nirmatrelvir-Ritonavir in Hospitalized Patients With COVID-19: A Retrospective Cohort Study, Journal of Medical Virology
Dian, Meng, Sun, Deng, Zeng, Azvudine Versus Paxlovid for Oral Treatment of COVID-19 in Chinese Patients With Pre-Existing Comorbidities, The Journal of Infection
Egger, Smith, Schneider, Minder, Bias in Meta-Analysis Detected by a Simple, Graphical Test, BMJ (Clinical Research Ed)
Gao, Luo, Ren, Antiviral Effect of Azvudine and Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19, The Journal of Infection
Hammond, Leister-Tebbe, Gardner, Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults With Covid-19, The New England Journal of Medicine
Han, Gao, Li, Real-World Effectiveness of Nirmatrelvir-Ritonavir Versus Azvudine in Hospitalized Patients With COVID-19 During the Omicron Wave in Beijing: A Multicenter Retrospective Cohort Study, BMC Infectious Diseases
Hu, Cui, Lei, Comparison of Azvudine and Nirmatrelvir/Ritonavir and Combined Use in Patients With COVID-19, Infection and Drug Resistance
Kumar, Delu, Shukla, Safety Assessment of a Nucleoside Analogue FNC (2′-Deoxy-2′-Beta-Fluoro-4′-Azidocytidine) in Balb/c Mice: Acute Toxicity Study, Asian Pacific Journal of Cancer Prevention
Lai, Shih, Ko, Tang, Hsueh, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges, International Journal of Antimicrobial Agents
Li, Zheng, Qi, Cui, Yang et al., A Retrospective Analysis of Azvudine in Patients With COVID-19 and Pre-Existing Cancer, Journal of Cancer
Liu, Yang, Xu, Azvudine and Mortality in Patients With Coronavirus Disease 2019: A Retrospective Cohort Study, International Immunopharmacology
Meng, Hong-Wen, Ze-Liang, Analysis of the Efficacy and Safety of Azvudine in Treating Moderate COVID-19 in Kidney Transplant Recipients, Chinese Journal of New Clinical Medicine
Schünemann, Cuello, Akl, GRADE Guidelines: 18. How ROBINS-I and Other Tools to Assess Risk of Bias in Nonrandomized Studies Should Be Used to Rate the Certainty of a Body of Evidence, Journal of Clinical Epidemiology
Shang, Fu, Geng, Azvudine Therapy of Common COVID-19 in Hemodialysis Patients, Journal of Medical Virology
Shao, Fan, Guo, Composite Interventions on Outcomes of Severely and Critically Ill Patients With COVID-19 in Shanghai, China, Microorganisms
Sterne, Egger, Funnel Plots for Detecting Bias in Meta-Analysis: Guidelines on Choice of Axis, Journal of Clinical Epidemiology
Sterne, Hernán, Reeves, ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions, BMJ (Clinical Research Ed)
Su, Yang, Wang, Azvudine Versus Paxlovid for Oral Treatment of COVID-19 in Chinese Patients, BMC Infectious Diseases
Sun, Jin, Dian, Oral Azvudine for Hospitalised Patients With COVID-19 and Pre-Existing Conditions: A Retrospective Cohort Study, EClinicalMedicine
Sun, Peng, Yu, Mechanistic Insight Into Antiretroviral Potency of 2'-Deoxy-2′-Beta-Fluoro-4′-Azidocytidine (FNC) With a Long-Lasting Effect on HIV-1 Prevention, Journal of Medicinal Chemistry
Wan, Wang, Liu, Tong, Estimating the Sample Mean and Standard Deviation From the Sample Size, Median, Range and/or Interquartile Range, BMC Medical Research Methodology
Wang, Mingzhen, Xiaojun, Peng, Clinical Effectiveness Evaluation of Azvudine in Mild and Moderate High-Risk Patients With COVlD-19 Infection, Chinese Journal of Hospital Pharmacy
Wang, Sun, Zhang, Antiviral Effectiveness and Survival Correlation of Azvudine and Nirmatrelvir/Ritonavir in Elderly Severe Patients With COVID-19: A Retrospective Real-World Study, EClinicalMedicine
Wang, Xie, Wang, Effectiveness of Azvudine in Reducing Mortality of COVID-19 Patients: A Systematic Review and Meta-Analysis, Virology Journal
Wei, Zeng, Wang, Head-to-Head Comparison of Azvudine and Nirmatrelvir/Ritonavir for the Hospitalized Patients With COVID-19: A Real-World Retrospective Cohort Study With Propensity Score Matching, Frontiers in Pharmacology
Wu, He, Huang, Azvudine for the Treatment of COVID-19 in Pre-Existing Cardiovascular Diseases: A Single-Center, Real-World Experience, Advanced Science
Yang, Wang, Jiang, Oral Azvudine for Mild-to-Moderate COVID-19 in High Risk, Nonhospitalized Adults: Results of a Real-World Study, Journal of Medical Virology
Yu, Chang, The First Chinese Oral Anti-COVID-19 Drug Azvudine Launched, Innovation (Camb)
Zhang, Li, Wang, Azvudine Is a Thymus-Homing Anti-SARS-CoV-2 Drug Effective in Treating COVID-19 Patients, Signal Transduction and Targeted Therapy
Zhang, Xiaojiao, Chen, Effectiveness of Nirmatrelvir-Ritonavir Versus Azvudine for Adult Inpatients With Severe or Critical COVID-19, BMJ Open Respiratory Research
Zhao, Cheng, Zhang, Efficacy of Nirmatrelvir-Ritonavir Versus Azvudine for COVID-19 Treatment in Tibet: A Retrospective Study, Infection and Drug Resistance
Zhao, Zheng, Han, Is Azvudine Comparable to Nirmatrelvir-Ritonavir in Real-World Efficacy and Safety for Hospitalized Patients With COVID-19? A Retrospective Cohort Study, Infectious Diseases and Therapy
Zhou, Liu, Jiang, Azvudine and Nirmatrelvir-Ritonavir in Hospitalized Patients With Moderate-to-Severe COVID-19: Emulation of a Randomized Target Trial, Journal of Medical Virology
Zhou, Zheng, Xiao, Xie, Rang et al., Effectiveness and Safety of Azvudine in Older Adults With Mild and Moderate COVID-19: A Retrospective Observational Study, BMC Infectious Diseases
Zhu, Efficacy and Safety Evaluation of Azvudine in the Prospective Treatment of COVID-19 Based on Four Phase III Clinical Trials, Frontiers in Pharmacology
Zong, Zhou, Li, Jiang, Liu et al., Azvudine Reduces the In-Hospital Mortality of COVID-19 Patients: A Retrospective Cohort Study, Acta Pharmaceutica Sinica B
DOI record:
{
"DOI": "10.1111/crj.13798",
"ISSN": [
"1752-6981",
"1752-699X"
],
"URL": "http://dx.doi.org/10.1111/crj.13798",
"abstract": "<jats:title>ABSTRACT</jats:title><jats:sec><jats:title>Background</jats:title><jats:p>Azvudine (FNC) is a novel small molecule antiviral drug for treating COVID‐19 that is available only on the Chinese market. Despite being recommended for treating COVID‐19 by the Chinese guidelines, its efficacy and safety are still unclear. This study aimed to evaluate the protective effect of FNC on COVID‐19 outcomes and its safety.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>We followed the PRISMA 2020 guidelines and searched the PubMed, Embase, Web of Science, Scopus, and China National Knowledge Infrastructure (CNKI) databases to evaluate studies on the effectiveness of FNC in treating COVID‐19 in China, focusing on mortality and overall outcomes. Additionally, its impact on the length of hospital stay (LOHS), time to first nucleic acid negative conversion (T‐FNANC), and adverse events was evaluated. The inclusion criterion was that the studies were published from July 2021 to April 10, 2024. This study uses the ROBINS‐I tool to assess bias risk and employs the GRADE approach to evaluate the certainty of the evidence.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The meta‐analysis included 24 retrospective studies involving a total of 11 830 patients. Low‐certainty evidence revealed no significant difference in mortality (OR = 0.91, 95% CI: 0.76–1.08) or LOHS (WMD = −0.24, 95% CI: −0.83 to 0.35) between FNC and Paxlovid in COVID‐19 patients. Low‐certainty evidence shows that the T‐FNANC was longer (WMD = 1.95, 95% CI: 0.36–3.53). Compared with the Paxlovid group, low‐certainty evidence shows the FNC group exhibited a worse composite outcome (OR = 0.77, 95% CI: 0.63–0.95) and fewer adverse events (OR = 0.63, 95% CI: 0.46–0.85). Compared with supportive treatment, low certainty shows FNC significantly reduced the mortality rate in COVID‐19 patients (OR = 0.61, 95% CI: 0.51–0.74) and decreased the composite outcome (OR = 0.67, 95% CI: 0.50–0.91), and very low certainty evidence shows significantly decreased the T‐FNANC (WMD = −4.62, 95% CI: −8.08 to −1.15). However, in very low certainty, there was no significant difference in LOHS (WMD = −0.70, 95% CI: −3.32 to 1.91) or adverse events (OR = 1.97, 95% CI: 0.48–8.17).</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>FNC appears to be a safe and potentially effective treatment for COVID‐19 in China, but further research with larger, high‐quality studies is necessary to confirm these findings. Due to the certainty of the evidence and the specific context of the studies conducted in China, caution should be exercised when considering whether the results are applicable worldwide.</jats:p></jats:sec><jats:sec><jats:title>Trial Registration</jats:title><jats:p>PROSPERO number: CRD42024520565</jats:p></jats:sec>",
"alternative-id": [
"10.1111/crj.13798"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2024-05-19"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "2024-06-02"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 3,
"value": "2024-07-12"
}
],
"author": [
{
"affiliation": [
{
"name": "Pharmacy Department Beijing Hospital of Integrated Traditional Chinese and Western Medicine Beijing China"
}
],
"family": "Dong",
"given": "Tao",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0009-0001-6067-8302",
"affiliation": [
{
"name": "Pharmacy Department Beijing Hospital of Integrated Traditional Chinese and Western Medicine Beijing China"
}
],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Wentao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Medical Device Monitoring and Evaluation Department National Center for ADR Monitoring Beijing China"
}
],
"family": "Wu",
"given": "Tingting",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Infectious Diseases Beijing Hospital of Integrated Chinese and Western Medicine Beijing China"
}
],
"family": "Ge",
"given": "Yongxiang",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Department Beijing Hospital of Integrated Traditional Chinese and Western Medicine Beijing China"
}
],
"family": "Yang",
"given": "Qi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Pharmacy Department Beijing Hospital of Integrated Traditional Chinese and Western Medicine Beijing China"
}
],
"family": "Xu",
"given": "Jia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Laboratory Beijing Hospital of Integrated Chinese and Western Medicine Beijing China"
}
],
"family": "Liu",
"given": "Yuna",
"sequence": "additional"
}
],
"container-title": "The Clinical Respiratory Journal",
"container-title-short": "Clinical Respiratory J",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2024,
7,
12
]
],
"date-time": "2024-07-12T09:39:35Z",
"timestamp": 1720777175000
},
"deposited": {
"date-parts": [
[
2024,
7,
29
]
],
"date-time": "2024-07-29T07:19:25Z",
"timestamp": 1722237565000
},
"indexed": {
"date-parts": [
[
2024,
7,
30
]
],
"date-time": "2024-07-30T00:16:38Z",
"timestamp": 1722298598208
},
"is-referenced-by-count": 0,
"issue": "7",
"issued": {
"date-parts": [
[
2024,
7
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2024,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 11,
"start": {
"date-parts": [
[
2024,
7,
12
]
],
"date-time": "2024-07-12T00:00:00Z",
"timestamp": 1720742400000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1111/crj.13798",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1111",
"published": {
"date-parts": [
[
2024,
7
]
]
},
"published-online": {
"date-parts": [
[
2024,
7,
12
]
]
},
"published-print": {
"date-parts": [
[
2024,
7
]
]
},
"publisher": "Wiley",
"reference": [
{
"DOI": "10.1016/j.ijantimicag.2020.105924",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_2_1"
},
{
"DOI": "10.1056/NEJMoa2118542",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_3_1"
},
{
"DOI": "10.1021/acs.jmedchem.0c00940",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_4_1"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_5_1"
},
{
"DOI": "10.31557/APJCP.2023.24.6.2157",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_6_1"
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_7_1"
},
{
"DOI": "10.3389/fphar.2023.1228548",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_8_1"
},
{
"DOI": "10.1016/S0895-4356(01)00377-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_9_1"
},
{
"DOI": "10.1136/bmj.315.7109.629",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_10_1"
},
{
"DOI": "10.1186/1471-2288-14-135",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_11_1"
},
{
"DOI": "10.1186/s12879-023-08944-z",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_12_1"
},
{
"DOI": "10.1186/s12879-023-08828-2",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_13_1"
},
{
"DOI": "10.1186/s12879-023-08965-8",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_14_1"
},
{
"DOI": "10.1002/jmv.29318",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_15_1"
},
{
"DOI": "10.1016/j.apsb.2023.07.007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_16_1"
},
{
"DOI": "10.3389/fphar.2023.1274294",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_17_1"
},
{
"DOI": "10.2147/IDR.S423725",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_18_1"
},
{
"DOI": "10.1002/jmv.29007",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_19_1"
},
{
"DOI": "10.1016/j.intimp.2023.110824",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_20_1"
},
{
"DOI": "10.1002/jmv.28947",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_21_1"
},
{
"DOI": "10.1016/j.jinf.2023.05.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_22_1"
},
{
"DOI": "10.1007/s40121-023-00845-7",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_23_1"
},
{
"DOI": "10.3390/microorganisms11071859",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_24_1"
},
{
"DOI": "10.1016/j.jinf.2023.03.023",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_25_1"
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_26_1"
},
{
"DOI": "10.1002/jmv.28756",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_27_1"
},
{
"DOI": "10.1101/2023.01.05.23284180",
"doi-asserted-by": "crossref",
"key": "e_1_2_10_28_1",
"unstructured": "W.Chen H.Xu L.Hong et al. “Oral Azvudine (FNC) Tablets in Patients Infected With SARS‐CoV‐2 Omicron Variant: A Retrospective Cohort Study ” In medRxiv; (2023)."
},
{
"article-title": "Analysis of the Efficacy and Safety of Azvudine in Treating Moderate COVID‐19 in Kidney Transplant Recipients",
"author": "Meng Y.",
"first-page": "1011",
"issue": "10",
"journal-title": "Chinese Journal of New Clinical Medicine",
"key": "e_1_2_10_29_1",
"volume": "16",
"year": "2023"
},
{
"article-title": "Clinical Effectiveness Evaluation of Azvudine in Mild and Moderate High‐Risk Patients With COVlD‐19 Infection",
"author": "Wang Z.",
"first-page": "2672",
"issue": "23",
"journal-title": "Chinese Journal of Hospital Pharmacy",
"key": "e_1_2_10_30_1",
"volume": "43",
"year": "2023"
},
{
"DOI": "10.7150/jca.91530",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_31_1"
},
{
"DOI": "10.1002/advs.202306050",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_32_1"
},
{
"DOI": "10.1016/j.eclinm.2024.102468",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_33_1"
},
{
"DOI": "10.1136/bmjresp-2023-001944",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_34_1"
},
{
"DOI": "10.2147/IDR.S433186",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_35_1"
},
{
"DOI": "10.1136/bmj.i4919",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_36_1"
},
{
"DOI": "10.1016/j.jclinepi.2018.01.012",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_37_1"
},
{
"DOI": "10.1186/s12985-024-02316-y",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_38_1"
},
{
"DOI": "10.1016/j.heliyon.2023.e20153",
"doi-asserted-by": "publisher",
"key": "e_1_2_10_39_1"
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1111/crj.13798"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy and Safety of Azvudine in Patients With COVID‐19 in China: A Meta‐Analysis of Observational Studies",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "18"
}
