Small-molecule antivirals treatment for COVID-19: A systematic review and network meta-analysis
et al., International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2024.107096, PROSPERO CRD42023396617, Jan 2024
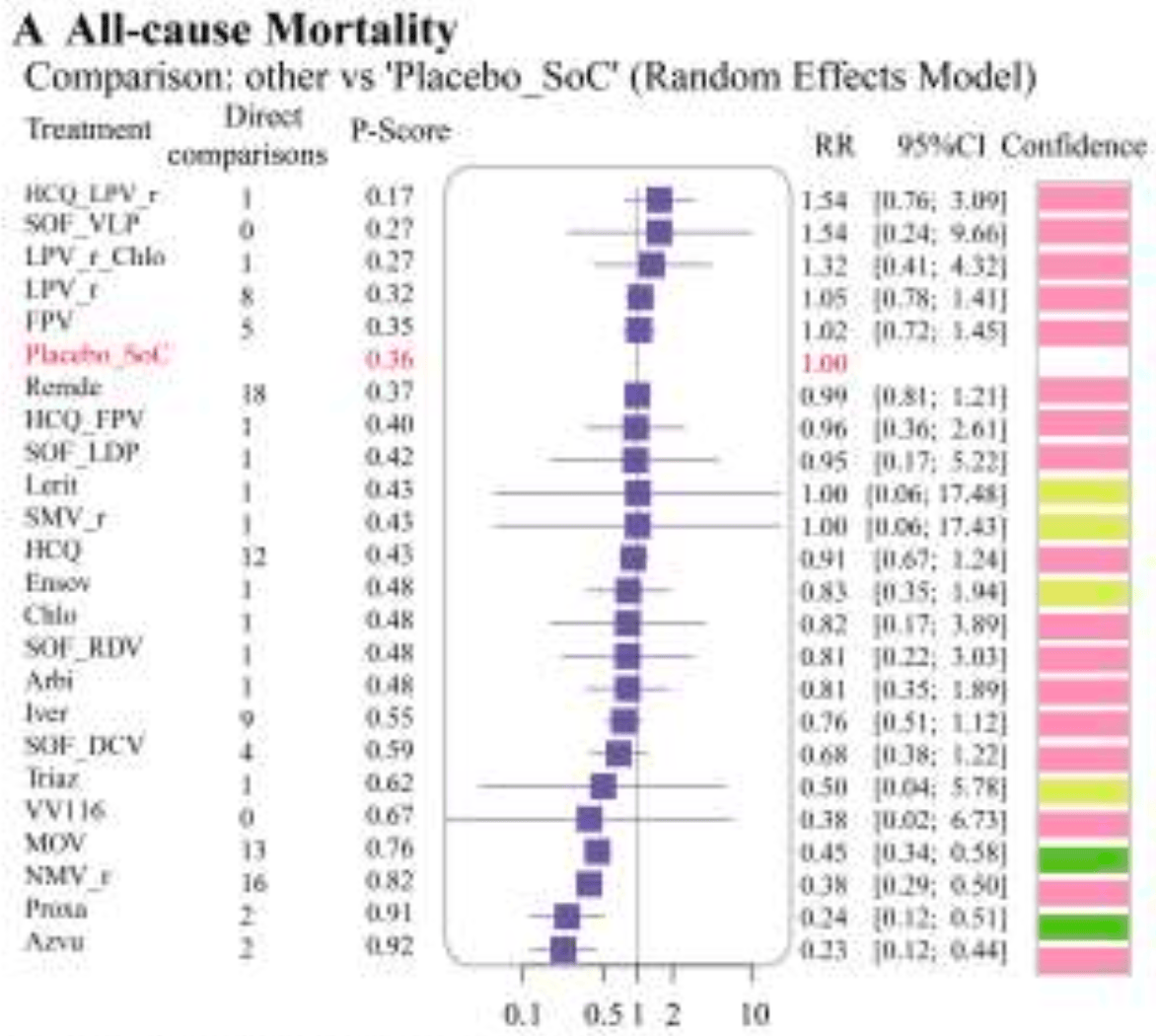
Systematic review and network meta-analysis of 160 studies involving over 900,000 COVID-19 patients assessing the efficacy and safety of small-molecule antivirals. For proxalutamide, significant benefits were found for mortality, mechanical ventilation, hospitalization, and clinical improvement.
Currently there are 4 proxalutamide studies and meta-analysis shows:
| Outcome | Improvement |
|---|---|
| Mortality | 78% lower [70‑83%] |
| Ventilation | 95% lower [60‑99%] |
| Hospitalization | 72% lower [19‑90%] |
Zheng et al., 18 Jan 2024, peer-reviewed, 11 authors, trial PROSPERO CRD42023396617.
Contact: 13819462633@163.com, zml9998@sina.com, ligonghua88@163.com.
Small-molecule antivirals treatment for COVID-19: A systematic review and network meta-analysis
International Journal of Antimicrobial Agents, doi:10.1016/j.ijantimicag.2024.107096
Highlights Systematic review and network meta-analysis assessing the efficacy and safety of small-molecule antivirals treatment for COVID-19. Proxalutamide, nirmatrelvir/ritonavir, triazavirin, azvudine, molnupiravir, and VV116 were ranked as the most effective drugs overall in both mild-to-moderate and unstratified groups. In both mild-to-moderate and unstratified groups, leritrelvir ranked first in virus clearance on days 7; however, no significant statistical difference was observed between leritrelvir and the subsequent medications Simnotrelvir/ritonavir and leritrelvir need further clinical data to confirm their efficacy and safety profiles. In terms of safety, the incidence of serious adverse events did not demonstrate an increase across all small-molecule antivirals, thereby confirming their favorable tolerability.
Competing Interests: The authors declare no competing interests. Ethical Approval: Not required.
Sequence Information: Not applicable
References
Ac Siemieniuk, Bartoszko, Zeraatkar, Drug treatments for covid-19: living systematic review and network meta-analysis, BMJ, doi:10.1136/bmj.m2980
Amani, Zareei, Amani, Rapid review and meta-analysis of adverse events associated with molnupiravir in patients with COVID-19, Br J Clin Pharmacol, doi:10.1111/bcp.15449
Behnam Amani, Amani, Efficacy and safety of nirmatrelvir/ritonavir (Paxlovid) for COVID-19: A rapid review and meta-analysis, Review J Med Virol, doi:10.1002/jmv.28441
Cheng, Chen, Jia, Efficacy and safety of current medications for treating severe and non-severe COVID-19 patients: an updated network meta-analysis of randomized placebo-controlled trials, Aging, doi:10.18632/aging.203522
Fariz, Zamzam Zein, Setiya Sulistiyana, Matthew Raffaello, Sofosbuvir with daclatasvir and the outcomes of patients with COVID-19: a systematic review and meta-analysis with GRADE assessment, Postgrad Med J, doi:10.1136/postgradmedj-2021-140287
Freeman, Fisher, White, Identifying inconsistency in network meta-analysis: is the net heat plot a reliable method?, Stat Med, doi:10.1002/sim.8383
Gao, Liu, Li, Molnupiravir for treatment of adults with mild or moderate COVID-19: a systematic review and meta-analysis of randomized controlled trials, Clin Microbiol Infect, doi:10.1016/j.cmi.2023.04.014
Gregori, Giacovelli, Minto, Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis A Systematic Review and Meta-analysis, JAMA, doi:10.1001/jama.2018.19319
Hannah E Davis, Mccorkell, Vogel, Long COVID: major findings, mechanisms and recommendations, Nat Rev Microbiol, doi:10.1038/s41579-022-00846-2
Higgins, Savović, Page, Chapter 8: Assessing risk of bias in a randomized trial In Cochrane Handbook for Systematic Reviews of Interventions Version 6.1
Hsu, Chen, Chen, Effect of sofosbuvir-based treatment on clinical outcomes of patients with COVID-19: a systematic review and meta-analysis of randomised controlled trials, Int J Antimicrob Agents, doi:10.1016/j.ijantimicag.2022.106545
Huzaifa A Cheema, Jafar, Sohail, Nirmatrelvir-ritonavir for the treatment of COVID-19 patients: A systematic review and meta-analysis, J Med Virol, doi:10.1002/jmv.28471
Javier, Sánchez, Martínez-Sellés, María Molero, García, Insights for COVID-19 in 2023, Rev Esp Quimioter, doi:10.37201/req/122.2022
Lancet, The COVID-19 pandemic in 2023: far from over, Lancet, doi:10.1016/S0140-6736(23)00050-8
Lei, Chen, Wu, Small molecules in the treatment of COVID-19, Signal Transduction and Targeted Therapy, doi:10.1038/s41392-022-01249-8
Li, He, Comparative efficacy and safety of current drugs against COVID-19: A systematic review and network meta-analysis, medRxiv, doi:10.1101/2020.11.16.20232884
Lo, Mertz, Loeb, Newcastle-Ottawa Scale: comparing reviewers' to authors' assessments, BMC Med Res Methodol, doi:10.1186/1471-2288-14-45
Page, Mckenzie, Bossuyt, The PRISMA 2020 statement: An updated guideline for reporting systematic reviews, Syst. Rev, doi:10.1136/bmj.n71
Peters, Sutton, Jones, Contouren hanced meta analysis funnel plots help distinguish publication bias from other causes of asymmetry, J Clin Epidemiol, doi:10.1016/j.jclinepi.2007.11.010
Pitre, Van Alstine, Chick, Antiviral drug treatment for nonsevere COVID-19: a systematic review and network meta-analysis, CMAJ, doi:10.1503/cmaj.220471
Polatoğlu, Oncu-Oner, Dalman, COVID-19 in early 2023: Structure, replication mechanism, variants of SARS-CoV-2, diagnostic tests, and vaccine & drug development studies, MedComm, doi:10.1002/mco2.228
Salanti, Ades, Ioannidis, Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial, J Clin Epidemiol, doi:10.1016/j.jclinepi.2010.03.016
Schandelmaier, Briel, Varadhan, Development of the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN) in randomized controlled trials and meta-analyses, CMAJ, doi:10.1503/cmaj.200077
Siang Kow, Javed, Ramachandram, Clinical outcomes of sofosbuvir-based antivirals in patients with COVID-19: a systematic review and meta-analysis of randomized trials, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2022.2000861
Unoh, Uehara, Nakahara, Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19, J. Med. Chem, doi:10.1021/acs.jmedchem.2c00117
Van Valkenhoef, Dias, Ades, Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis, Res Synth Methods, doi:10.1002/jrsm.1167
Veroniki, Straus, Fyraridis, The rank-heat plot is a novel way to present the results from a network meta-analysis including multiple outcomes, J Clin Epidemiol, doi:10.1016/j.jclinepi.2016.02.016
Veroniki, Vasiliadis, Higgins, Evaluation of inconsistency in networks of interventions, Int J Epidemiol, doi:10.1093/ije/dys222
Vivek P Chavda 1, Aayushi, Patel 2, Darsh, Vaghasiya, SARS-CoV-2 variants and vulnerability at the global level, J Med Virol, doi:10.1002/jmv.27717
Vivek P Chavda, Raval, Sheta, Blood filtering system for COVID-19 management: novel modality of the cytokine storm therapeutics, Front Immunol, doi:10.3389/fimmu.2023.1064459
Who, Coronavirus disease COVID-19
Zheng, Ma, Wang, Efficacy and safety of Paxlovid for COVID-19: a meta-analysis, J Infect, doi:10.1016/j.jinf.2022.09.027
DOI record:
{
"DOI": "10.1016/j.ijantimicag.2024.107096",
"ISSN": [
"0924-8579"
],
"URL": "http://dx.doi.org/10.1016/j.ijantimicag.2024.107096",
"alternative-id": [
"S0924857924000141"
],
"article-number": "107096",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Small-molecule antivirals treatment for COVID-19: A systematic review and network meta-analysis"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "International Journal of Antimicrobial Agents"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.ijantimicag.2024.107096"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2024 The Author(s). Published by Elsevier Ltd."
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0003-0575-1607",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zheng",
"given": "Bei",
"sequence": "first"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Qinqin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Wenjuan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feng",
"given": "Pinpin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xin",
"given": "Chuanwei",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4767-0602",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ying",
"given": "Yin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1572-9764",
"affiliation": [],
"authenticated-orcid": false,
"family": "Yang",
"given": "Bo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Han",
"given": "Bing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhu",
"given": "Jun",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1023-8827",
"affiliation": [],
"authenticated-orcid": false,
"family": "Zhang",
"given": "Meiling",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Li",
"given": "Gonghua",
"sequence": "additional"
}
],
"container-title": "International Journal of Antimicrobial Agents",
"container-title-short": "International Journal of Antimicrobial Agents",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.es",
"clinicalkey.com.au",
"clinicalkey.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
18
]
],
"date-time": "2024-01-18T17:06:20Z",
"timestamp": 1705597580000
},
"deposited": {
"date-parts": [
[
2024,
1,
19
]
],
"date-time": "2024-01-19T23:15:17Z",
"timestamp": 1705706117000
},
"funder": [
{
"DOI": "10.13039/501100020777",
"award": [
"2022ZYJ08",
"2022ZYJ20"
],
"doi-asserted-by": "publisher",
"name": "Zhejiang Pharmaceutical Association"
},
{
"DOI": "10.13039/501100004731",
"award": [
"LGF20G030004",
"LYQ20H310003"
],
"doi-asserted-by": "publisher",
"name": "Natural Science Foundation of Zhejiang Province"
}
],
"indexed": {
"date-parts": [
[
2024,
1,
20
]
],
"date-time": "2024-01-20T23:11:56Z",
"timestamp": 1705792316721
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
1
]
],
"date-time": "2024-01-01T00:00:00Z",
"timestamp": 1704067200000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 16,
"start": {
"date-parts": [
[
2024,
1,
17
]
],
"date-time": "2024-01-17T00:00:00Z",
"timestamp": 1705449600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857924000141?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S0924857924000141?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "107096",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2024,
1
]
]
},
"published-print": {
"date-parts": [
[
2024,
1
]
]
},
"publisher": "Elsevier BV",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S0924857924000141"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Microbiology (medical)",
"General Medicine"
],
"subtitle": [],
"title": "Small-molecule antivirals treatment for COVID-19: A systematic review and network meta-analysis",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy"
}