Efficacy of Azvudine Therapy in Patients with Severe and Non-Severe COVID‐19: A Propensity Score-Matched Analysis
et al., Infection and Drug Resistance, doi:10.2147/IDR.S481591, Oct 2024
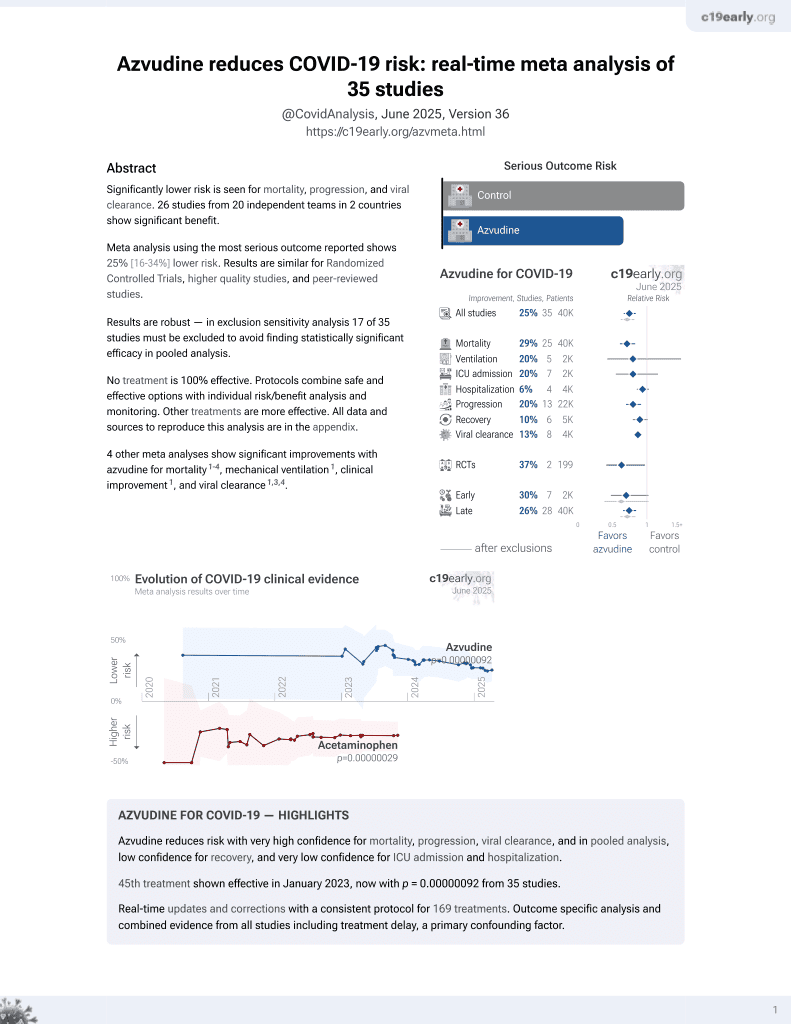
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
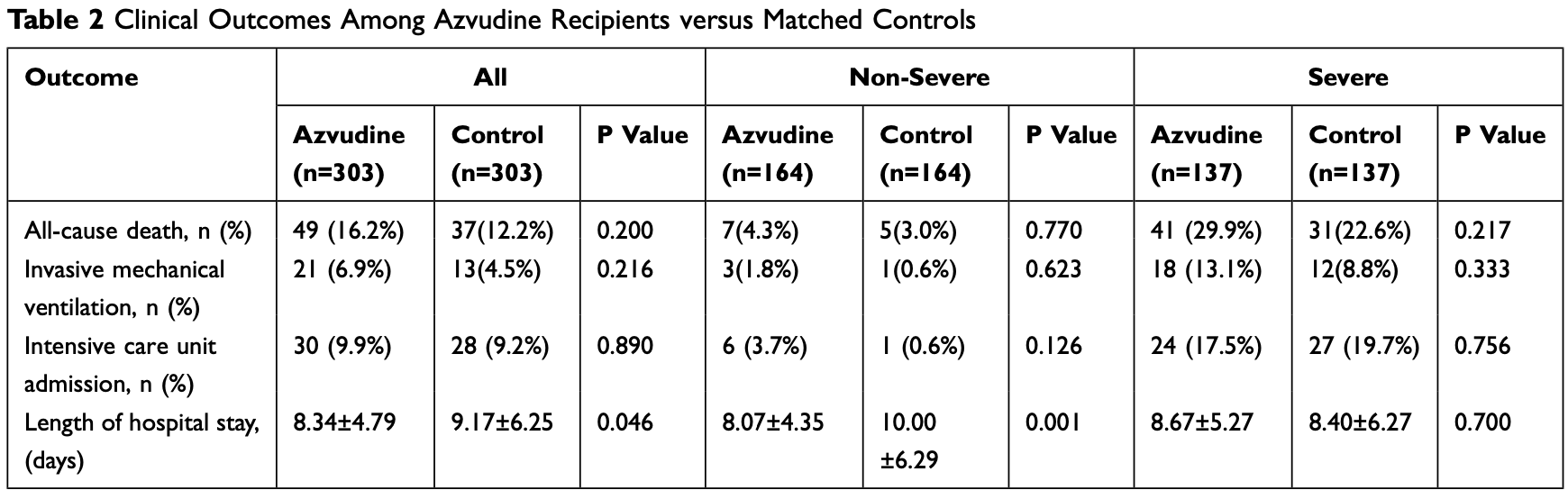
PSM retrospective 303 hospitalized patients treated with azvudine and 303 matched controls in China, showing shorter hospital stay and higher lymphocyte improvement rate, particularly for non-severe patients, however there were no significant differences for mortality, ICU admission, or mechanical ventilation.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments3.
|
risk of death, 32.4% higher, RR 1.32, p = 0.20, treatment 49 of 303 (16.2%), control 37 of 303 (12.2%).
|
|
risk of mechanical ventilation, 61.5% higher, RR 1.62, p = 0.22, treatment 21 of 303 (6.9%), control 13 of 303 (4.3%).
|
|
risk of ICU admission, 7.1% higher, RR 1.07, p = 0.89, treatment 30 of 303 (9.9%), control 28 of 303 (9.2%).
|
|
hospitalization time, 9.1% lower, relative time 0.91, p = 0.046, treatment mean 8.34 (±4.79) n=303, control mean 9.17 (±6.25) n=303, all patients.
|
|
hospitalization time, 19.3% lower, relative time 0.81, p = 0.001, treatment mean 8.07 (±4.35) n=165, control mean 10.0 (±6.29) n=181, non-severe patients.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Xiong et al., Real-world data of Azvudine-induced hepatotoxicity among hospitalized COVID-19 patients in China: a retrospective case-control study, Frontiers in Pharmacology, doi:10.3389/fphar.2025.1558054.
Zhang et al., 7 Oct 2024, retrospective, China, peer-reviewed, mean age 68.8, 7 authors, study period 10 December, 2022 - 10 January, 2023.
Contact: liulin0956@163.com.
Efficacy of Azvudine Therapy in Patients with Severe and Non-Severe COVID‐19: A Propensity Score-Matched Analysis
Infection and Drug Resistance, doi:10.2147/idr.s481591
Objective: Azvudine is used to treat patients with the coronavirus disease 2019 (COVID-19). This study evaluated the clinical efficacy of azvudine in hospitalized patients with different severities of COVID-19 because few studies have described this in patients with severe and non-severe COVID-19. Methods: This retrospective study included hospitalized patients with COVID-19 in Guizhou Provincial People's Hospital between December 2022 and January 2023. Azvudine-treated patients and controls were matched for sex, age, and disease severity at admission. Laboratory results and outcomes, including all-cause mortality, invasive mechanical ventilation, intensive care unit admission, and hospital stay length, were evaluated. Stratified analysis was used to explore the difference in the efficacy of azvudine in severe and non-severe COVID-19 patients. Results: No significant differences in all-cause mortality were observed between the 303 azvudine recipients and 303 matched controls. However, azvudine-treated patients had shorter hospital stays (8.34±4.79 vs 9.17±6.25 days, P=0.046) and higher lymphocyte improvement rates (21.5% vs 13.9%, P=0.019), with a more pronounced effect in patients with non-severe COVID-19 (length of hospital stay, 8.07±4.35 vs 10.00±6.29 days, P=0.001; lymphocyte improvement rate, 23.8% vs 12.8%, P=0.015). Conclusion: Azvudine treatment shortens hospital stay length and increases the rate of lymphocyte count improvement in patients with non-severe COVID-19, suggesting that azvudine may be a treatment option for these patients.
Ethics Approval and Informed Consent This study was conducted in accordance with the Declaration of Helsinki, and was approved by the Ethics Committee of Guizhou Provincial People's Hospital (No. [2022] 113). Due to the retrospective design and the use of anonymized data, the requirement for informed consent was waived.
Author Contributions All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure The authors report no conflicts of interest in this work.
References
Chang, 4′-Modified Nucleosides for Antiviral Drug Discovery: achievements and Perspectives, Acc Chem Res, doi:10.1021/acs.accounts.1c00697
Chen, Lin, Lu, Real-world effectiveness of molnupiravir, azvudine and paxlovid against mortality and viral clearance among hospitalized patients with COVID-19 infection during the omicron wave in China: a retrospective cohort study, Diagn Microbiol Infect Dis, doi:10.1016/j.diagmicrobio.2024.116353
Chen, Tian, Efficacy and safety of azvudine in patients with COVID-19: a systematic review and meta-analysis, Heliyon, doi:10.1016/j.heliyon.2023.e20153
Da Silva, Cabral, Souza, Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front Med, doi:10.3389/fmed.2023.1143485
De Souza, Cabral, Da Silva, Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients, Front Med, doi:10.3389/fmed.2023.1215916
Gudima, Kofiadi, Shilovskiy, Antiviral Therapy of COVID-19, Int J Mol Sci, doi:10.3390/ijms24108867
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Ioannidis, Zonta, Levitt, Estimates of COVID-19 deaths in Mainland China after abandoning zero COVID policy, medRxiv
Kapar, Xie, Guo, Effectiveness of azvudine against severe outcomes among hospitalized COVID-19 patients in Xinjiang, China: a single-center, retrospective, matched cohort study, Expert Rev Anti Infect Ther, doi:10.1080/14787210.2024.2362900
Liao, Liang, Chen, IL-19 induces production of IL-6 and TNF-alpha and results in cell apoptosis through TNF-alpha, J Immunol, doi:10.4049/jimmunol.169.8.4288
Liu, Yang, Xu, Azvudine and mortality in patients with coronavirus disease 2019: a retrospective cohort study, Int Immunopharmacol, doi:10.1016/j.intimp.2023.110824
Luo, Wang, Wu, Peripheral Lymphocyte Count and Viral Clearance in COVID-19, J Coll Physicians Surg Pak
Mazzitelli, Mengato, Sasset, Molnupiravir and Nirmatrelvir/Ritonavir: tolerability, Safety, and Adherence in a Retrospective Cohort Study, Viruses, doi:10.3390/v15020384
Murakami, Hayden, Hills, Therapeutic advances in COVID-19, Nat Rev Nephrol, doi:10.1038/s41581-022-00642-4
Paton, Overton, Ward, The rapid replacement of the SARS-CoV-2 Delta variant by Omicron (B.1.1.529) in England, Sci Transl Med, doi:10.1126/scitranslmed.abo5395
Planas, Saunders, Maes, Considerable escape of SARS-CoV-2 Omicron to antibody neutralization, Nature, doi:10.1038/s41586-021-04389-z
Ren, Luo, Yu, A Randomized, Open-Label, Controlled Clinical Trial of Azvudine Tablets in the Treatment of Mild and Common COVID-19, a Pilot Study, Adv Sci, doi:10.1002/advs.202001435
Richards, Kodjamanova, Chen, Economic Burden of COVID-19: a Systematic Review, Clinicoecon Outcomes Res, doi:10.2147/CEOR.S338225
Shang, Fu, Geng, Azvudine therapy of common COVID-19 in hemodialysis patients, J Med Virol, doi:10.1002/jmv.29007
Shao, Fan, Guo, Composite Interventions on Outcomes of Severely and Critically Ill Patients with COVID-19 in Shanghai, China, Microorganisms, doi:10.3390/microorganisms11071859
Sheng, Li, Li, Selectively T cell phosphorylation activation of azvudine in the thymus tissue with immune protection effect, Acta Pharm Sin B, doi:10.1016/j.apsb.2024.03.032
Singh, De, Antiviral agents for the treatment of COVID-19: progress and challenges, Cell Rep Med, doi:10.1016/j.xcrm.2022.100549
Sun, Dian, Oral Azvudine for hospitalised patients with COVID-19 and pre-existing conditions: a retrospective cohort study, EClinicalMedicine, doi:10.1016/j.eclinm.2023.101981
Sun, Peng, Yu, Mechanistic Insight into Antiretroviral Potency of 2'-Deoxy-2'-beta-fluoro-4'-azidocytidine (FNC) with a Long-Lasting Effect on HIV-1 Prevention, J Med Chem, doi:10.1021/acs.jmedchem.0c00940
Terpos, Ntanasis-Stathopoulos, Elalamy, Hematological findings and complications of COVID-19, Am J Hematol, doi:10.1002/ajh.25829
Wang, Hu, Hu, Clinical Characteristics of 138 hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Wang, Xie, Wang, Effectiveness of azvudine in reducing mortality of COVID-19 patients: a systematic review and meta-analysis, Virol J, doi:10.1186/s12985-024-02316-y
Xu, Yang, Zheng, The Pyrimidine Analog FNC Potently Inhibits the Replication of Multiple Enteroviruses, J Virol, doi:10.1128/JVI.00204-20
Xu, Zhong, Deng, High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa, Int J Oral Sci, doi:10.1038/s41368-020-0074-x
Yang, Wang, Bench-to-bedside: innovation of small molecule anti-SARS-CoV-2 drugs in China, Eur J Med Chem, doi:10.1016/j.ejmech.2023.115503
Yang, Wang, Jiang, Oral azvudine for mild-to-moderate COVID-19 in high risk, nonhospitalized adults: results of a real-world study, J Med Virol, doi:10.1002/jmv.28947
Yang, Wang, Wang, epidemiology of antibiotic resistance and the mechanisms of resistance development and diffusion in both hospitals and the community, doi:10.1016/j.clinthera.2024.07.009
Yang, Zhang, Wang, Adherence and recommended optimal treatment to Azvudine application for the treatment of outpatient COVID-19 patients: a real-world retrospective study, Heliyon, doi:10.1016/j.heliyon.2024.e30619
Yu, Chang, Azvudine (FNC): a promising clinical candidate for COVID-19 treatment, Signal Transduct Target Ther, doi:10.1038/s41392-020-00351-z
Yu, Chang, The first Chinese oral anti-COVID-19 drug Azvudine launched, Innovation, doi:10.1016/j.xinn.2022.100321
Zhang, Li, Wang, Azvudine is a thymus-homing anti-SARS-CoV-2 drug effective in treating COVID-19 patients, Signal Transduct Target Ther, doi:10.1038/s41392-021-00835-6
Zhao, Meng, Kumar, Lymphopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a systemic review and meta-analysis, Int J Infect Dis, doi:10.1016/j.ijid.2020.04.086
DOI record:
{
"DOI": "10.2147/idr.s481591",
"ISSN": [
"1178-6973"
],
"URL": "http://dx.doi.org/10.2147/IDR.S481591",
"author": [
{
"ORCID": "http://orcid.org/0009-0009-0174-8065",
"affiliation": [],
"authenticated-orcid": true,
"family": "Zhang",
"given": "Siqin",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0009-0000-2259-9059",
"affiliation": [],
"authenticated-orcid": true,
"family": "Tan",
"given": "Songsong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Bin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wu",
"given": "Yaoyao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yuan",
"given": "Guohang",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Fengjiao",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-1947-0809",
"affiliation": [],
"authenticated-orcid": true,
"family": "Liu",
"given": "Lin",
"sequence": "additional"
}
],
"container-title": "Infection and Drug Resistance",
"container-title-short": "IDR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
10,
7
]
],
"date-time": "2024-10-07T04:10:17Z",
"timestamp": 1728274217000
},
"deposited": {
"date-parts": [
[
2024,
10,
7
]
],
"date-time": "2024-10-07T04:10:23Z",
"timestamp": 1728274223000
},
"indexed": {
"date-parts": [
[
2024,
10,
8
]
],
"date-time": "2024-10-08T04:03:16Z",
"timestamp": 1728360196880
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
10
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
1
]
],
"date-time": "2024-10-01T00:00:00Z",
"timestamp": 1727740800000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/article/download/96218",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/article/download/96218",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "4317-4325",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2024,
10
]
]
},
"published-online": {
"date-parts": [
[
2024,
10
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1016/S0140-6736(22)00484-6",
"author": "Collaborators",
"doi-asserted-by": "publisher",
"first-page": "2351",
"journal-title": "Lancet",
"key": "ref1",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.2147/CEOR.S338225",
"author": "Richards",
"doi-asserted-by": "publisher",
"first-page": "293",
"journal-title": "Clinicoecon Outcomes Res",
"key": "ref2",
"volume": "14",
"year": "2022"
},
{
"author": "Ioannidis",
"first-page": "1",
"journal-title": "medRxiv",
"key": "ref3",
"volume": "2023",
"year": "2023"
},
{
"DOI": "10.1126/scitranslmed.abo5395",
"author": "Paton",
"doi-asserted-by": "publisher",
"first-page": "eabo5395",
"journal-title": "Sci Transl Med",
"key": "ref4",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"author": "Planas",
"doi-asserted-by": "publisher",
"first-page": "671",
"journal-title": "Nature",
"key": "ref5",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.3390/ijms24108867",
"author": "Gudima",
"doi-asserted-by": "publisher",
"first-page": "8867",
"journal-title": "Int J Mol Sci",
"key": "ref6",
"volume": "24",
"year": "2023"
},
{
"DOI": "10.1016/j.xcrm.2022.100549",
"author": "Singh",
"doi-asserted-by": "publisher",
"first-page": "100549",
"journal-title": "Cell Rep Med",
"key": "ref7",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1038/s41581-022-00642-4",
"author": "Murakami",
"doi-asserted-by": "publisher",
"first-page": "38",
"journal-title": "Nat Rev Nephrol",
"key": "ref8",
"volume": "19",
"year": "2023"
},
{
"DOI": "10.3390/v15020384",
"author": "Mazzitelli",
"doi-asserted-by": "publisher",
"first-page": "384",
"journal-title": "Viruses",
"key": "ref9",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1016/j.xinn.2022.100321",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "100321",
"journal-title": "Innovation",
"key": "ref10",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1128/JVI.00204-20",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "e00204",
"journal-title": "J Virol",
"key": "ref11",
"volume": "94",
"year": "2020"
},
{
"DOI": "10.1021/acs.jmedchem.0c00940",
"author": "Sun",
"doi-asserted-by": "publisher",
"first-page": "8554",
"journal-title": "J Med Chem",
"key": "ref12",
"volume": "63",
"year": "2020"
},
{
"DOI": "10.1021/acs.accounts.1c00697",
"author": "Chang",
"doi-asserted-by": "publisher",
"first-page": "565",
"journal-title": "Acc Chem Res",
"key": "ref13",
"volume": "55",
"year": "2022"
},
{
"DOI": "10.1038/s41392-020-00351-z",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "236",
"journal-title": "Signal Transduct Target Ther",
"key": "ref14",
"volume": "5",
"year": "2020"
},
{
"DOI": "10.1016/j.ejmech.2023.115503",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "115503",
"journal-title": "Eur J Med Chem",
"key": "ref15",
"volume": "257",
"year": "2023"
},
{
"DOI": "10.3389/fmed.2023.1143485",
"author": "da Silva",
"doi-asserted-by": "publisher",
"first-page": "1143485",
"journal-title": "Front Med",
"key": "ref16",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1016/j.heliyon.2023.e20153",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "e20153",
"journal-title": "Heliyon",
"key": "ref17",
"volume": "9",
"year": "2023"
},
{
"DOI": "10.1002/advs.202001435",
"author": "Ren",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"journal-title": "Adv Sci",
"key": "ref18",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1186/s12985-024-02316-y",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "46",
"journal-title": "Virol J",
"key": "ref19",
"volume": "21",
"year": "2024"
},
{
"DOI": "10.3389/fmed.2023.1215916",
"author": "de Souza",
"doi-asserted-by": "publisher",
"first-page": "1215916",
"journal-title": "Front Med",
"key": "ref20",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1002/jmv.28947",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "e28947",
"journal-title": "J Med Virol",
"key": "ref21",
"volume": "95",
"year": "2023"
},
{
"key": "ref22",
"unstructured": "General Office of the National Health Commission. Diagnosis and treatment protocol for COVID-19 in China (trial version 10). Available from: http://www.gov.cn/zhengce/zhengceku/2023-01/06/content_5735343.htm. 2023, Accessed February 1, 2024."
},
{
"DOI": "10.1016/j.eclinm.2023.101981",
"author": "Sun",
"doi-asserted-by": "publisher",
"first-page": "101981",
"journal-title": "EClinicalMedicine",
"key": "ref23",
"volume": "59",
"year": "2023"
},
{
"DOI": "10.1016/j.intimp.2023.110824",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "110824",
"journal-title": "Int Immunopharmacol",
"key": "ref24",
"volume": "124",
"year": "2023"
},
{
"DOI": "10.1016/j.diagmicrobio.2024.116353",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "116353",
"journal-title": "Diagn Microbiol Infect Dis",
"key": "ref25",
"volume": "109",
"year": "2024"
},
{
"DOI": "10.1080/14787210.2024.2362900",
"author": "Kapar",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Expert Rev Anti Infect Ther",
"key": "ref26",
"year": "2024"
},
{
"DOI": "10.3390/microorganisms11071859",
"author": "Shao",
"doi-asserted-by": "publisher",
"first-page": "1859",
"journal-title": "Microorganisms",
"key": "ref27",
"volume": "11",
"year": "2023"
},
{
"DOI": "10.1002/jmv.29007",
"author": "Shang",
"doi-asserted-by": "publisher",
"first-page": "e29007",
"journal-title": "J Med Virol",
"key": "ref28",
"volume": "95",
"year": "2023"
},
{
"DOI": "10.1038/s41392-021-00835-6",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "414",
"journal-title": "Signal Transduct Target Ther",
"key": "ref29",
"volume": "6",
"year": "2021"
},
{
"DOI": "10.1016/j.apsb.2024.03.032",
"author": "Sheng",
"doi-asserted-by": "publisher",
"first-page": "3140",
"journal-title": "Acta Pharm Sin B",
"key": "ref30",
"volume": "14",
"year": "2024"
},
{
"DOI": "10.1016/j.ijid.2020.04.086",
"author": "Zhao",
"doi-asserted-by": "publisher",
"first-page": "131",
"journal-title": "Int J Infect Dis",
"key": "ref31",
"volume": "96",
"year": "2020"
},
{
"DOI": "10.1038/s41368-020-0074-x",
"author": "Xu",
"doi-asserted-by": "publisher",
"first-page": "8",
"journal-title": "Int J Oral Sci",
"key": "ref32",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.169.8.4288",
"author": "Liao",
"doi-asserted-by": "publisher",
"first-page": "4288",
"journal-title": "J Immunol",
"key": "ref33",
"volume": "169",
"year": "2002"
},
{
"DOI": "10.1002/ajh.25829",
"author": "Terpos",
"doi-asserted-by": "publisher",
"first-page": "834",
"journal-title": "Am J Hematol",
"key": "ref34",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "1061",
"journal-title": "JAMA",
"key": "ref35",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet",
"key": "ref36",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.29271/jcpsp.2022.12.1637",
"author": "Luo",
"doi-asserted-by": "crossref",
"first-page": "1637",
"journal-title": "J Coll Physicians Surg Pak",
"key": "ref37",
"volume": "32",
"year": "2022"
},
{
"DOI": "10.1016/j.heliyon.2024.e30619",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "e30619",
"journal-title": "Heliyon",
"key": "ref38",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1016/j.clinthera.2024.07.009",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "e1",
"journal-title": "Clin Ther",
"key": "ref39",
"volume": "46",
"year": "2024"
}
],
"reference-count": 39,
"references-count": 39,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/efficacy-of-azvudine-therapy-in-patients-with-severe-and-non-severe-co-peer-reviewed-fulltext-article-IDR"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficacy of Azvudine Therapy in Patients with Severe and Non-Severe COVID‐19: A Propensity Score-Matched Analysis",
"type": "journal-article",
"volume": "Volume 17"
}