Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients
et al., Frontiers in Medicine, doi:10.3389/fmed.2023.1215916, NCT04668235, Oct 2023
Azvudine for COVID-19
48th treatment shown to reduce risk in
January 2023, now with p = 0.0000000041 from 40 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 179 hospitalized patients in Brazil, showing improved recovery with azvudine treatment.
|
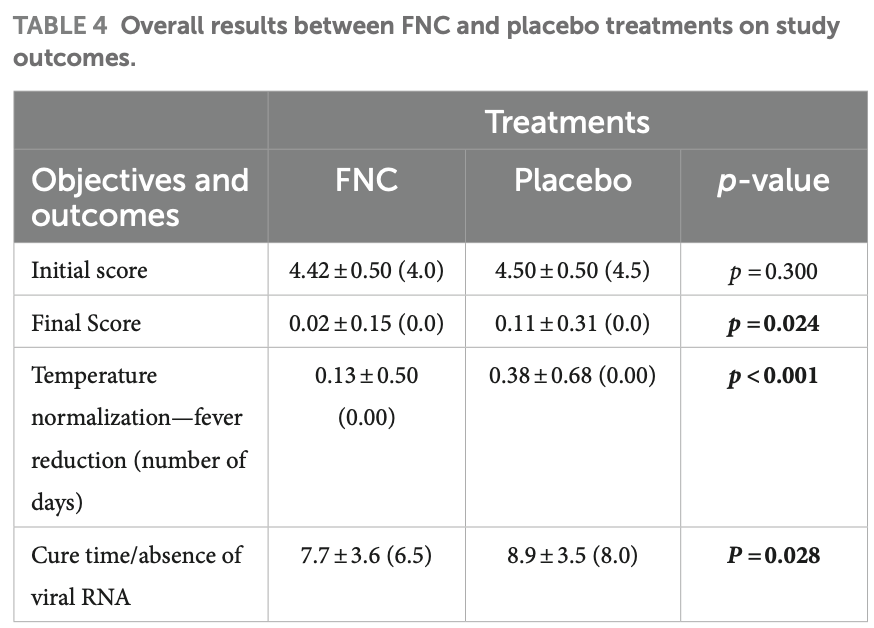
risk of ICU admission, 27.5% lower, RR 0.73, p = 0.72, treatment 3 of 91 (3.3%), control 4 of 88 (4.5%), NNT 80.
|
|
risk of no hospital discharge, 42.0% lower, RR 0.58, p = 0.49, treatment 3 of 91 (3.3%), control 5 of 88 (5.7%), NNT 42.
|
|
relative final score, 81.8% better, RR 0.18, p = 0.01, treatment mean 0.02 (±0.15) n=91, control mean 0.11 (±0.31) n=88.
|
|
time to viral-, 13.0% lower, relative time 0.87, p = 0.03, treatment 91, control 88.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
de Souza et al., 19 Oct 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, median age 48.0, 12 authors, study period April 2021 - May 2022, trial NCT04668235 (history).
Contact: saviobas@gmail.com, pgacabral99@gmail.com, 002001@htu.edu.cn.
Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients
Frontiers in Medicine, doi:10.3389/fmed.2023.1215916
Background: In 2019, a highly pathogenic coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) surfaced and resulted in the outbreak of coronavirus disease 2019 . With the aim of finding effective drugs to fight against the disease, several trials have been conducted since COVID-19 can only be considered a treatable disease, from a clinical point of view, after the availability of specific and effective antivirals. AZVUDINE (FNC), initially developed for treating HIV, is a potential treatment for COVID-19 as it has the capability to lower the patient's viral load and promote recovery. Methods: Volunteers infected with SARS-CoV-2 confirmed by reverse transcription polymerase chain reaction (RT-PCR), with good kidney and liver function, who were not using other antivirals or monoclonal antibodies were eligible. Samples from patients were assessed for viral load every 48 h during treatment using reverse transcription quantitative polymerase chain reaction (RT-qPCR) and droplet digital polymerase chain reaction (ddPCR). Results: The study's primary outcome measure was the percentage of participants showing an improvement in clinical scores, while the secondary outcome measure was the percentage of participants with a clinical outcome of cure. These measures were used to assess the safety and efficacy of FNC for treating COVID-19. In the analysis of sociodemographic variables, no significant differences were detected between patients in the FNC and the placebo group for race, age group, or sex. The results showed a potential benefit to participants who received FNC during the study, as observed in the shorter hospital stay, shorter negative conversion time of SARS-CoV-2, and a significant reduction in viral load. Furthermore, the reduction in fever and chills were significant at D1, D2, and D3.
Statistical analysis Initially, there were 342 participants in the study. However, due to the decrease in the number of COVID-19 cases in Brazil toward the end of 2021, the sample size was reevaluated and subsequently reduced to 180 participants. These participants were randomly assigned to two study groups, each consisting of 90 participants. All enrolled patients with moderate COVID-19 were hospitalized. The sample calculation was performed using the formula of "sample calculation for superiority studies using proportions, " described by World Health Organization (14) . To analyze demographic information and baseline eigenvalues, descriptive statistics such as mean, standard deviation, quartiles, and minimum and maximum values were calculated for numerical variables. Frequency and percentage were determined for categorical data. The appropriate statistical methods were employed to compare the two groups based on the type of indicator. The Mann-Whitney test was utilized to compare quantitative data, while Fisher's exact test was employed for categorical data. All statistical analyses were conducted using R-studio software.
Quantification of SARS-CoV-2 viral load by reverse transcription-polymerase chain reaction The MagMAXTM Viral/Pathogen Nucleic Acid Isolation kit (Applied Biosystems) was employed to extract total RNA from nasal and throat swabs obtained from the participants of the clinical study. The extraction process was carried out in accordance with the..
References
Abdelnabi, Foo, Kaptein, Zhang, Langendries et al., The combined treatment of Molnupiravir and Favipiravir results in a marked potentiation of efficacy in a SARS-CoV2 hamster infection model through an in-creased frequency of mutations in the viral genome, bioRxiv, doi:10.1101/2020.12.10.419242
Ackley, Mcmanus, Topal, Cicali, Shah, A valid warning or clinical Lore: an evaluation of safety outcomes of Remdesivir in patients with impaired renal function from a multicenter matched cohort, Antimicrob Agents Chemother, doi:10.1128/aac.02290-20
Adamsick, Gandhi, Bidell, Elshaboury, Bhattacharyya et al., Remdesivir in patients with acute or chronic kidney disease and COVID-19, J Am Soc Nephrol, doi:10.1681/ASN.2020050589
Ahmad, Kiani, Abrar, Zafar, Ali, A comprehensive genomic study, mutation screening, phylogenetic and statistical analysis of SARS-CoV-2 and its variant omicron among different countries, J Infect Public Health, doi:10.1016/j.jiph.2022.07.002
Beigel, Tomashek, Dodd, Mehta, Zingman et al., Remdesivir for the treatment of Covid-19-final report, N Engl J Med, doi:10.1056/NEJMoa2007764
Da Silva, Abreu Cabral, De Souza, Arruda, Cabral et al., Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19, Front Med, doi:10.3389/fmed.2023.1143485
Fajnzylber, Regan, Coxen, Corry, Wong et al., SARS-CoV-2 viral load is associated with increased disease severity and mortality, Nat Commun, doi:10.1038/s41467-020-19057-5
Fodha, Vabret, Ghedira, Seboui, Chouchane et al., Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity, J Med Virol, doi:10.1002/jmv.21026
Gupta, Mccoy, Mok, Peppa, Salgado, HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation, Nature, doi:10.1038/s41586-019-1027-4
Huang, Liu, Wang, Xu, Yang et al., Next generation digital PCR measurement of hepatitis B virus copy number in formalin-fixed paraffinembedded hepatocellular carcinoma tissue, Clin Chem, doi:10.1373/clinchem.2014.230227
Jordheim, Durantel, Zoulim, Dumontet, Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases, Nat Rev Drug Discov, doi:10.1038/nrd4010
Liu, Yan, Xiang, Le, Liu, Viral dynamics in mild and severe cases of COVID-19, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30232-2
Magleby, Westblade, Trzebucki, Simon, Rajan et al., Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019, Clin Infect Dis, doi:10.1093/cid/ciaa851
Pan, Zhang, Yang, Poon, Wang, Viral load of SARS-CoV-2 in clinical samples, Lancet Infect Dis, doi:10.1016/S1473-3099(20)30113-4
Pujadas, Chaudhry, Mcbride, Richter, Zhao et al., SARS-CoV-2 viral load predicts COVID-19 mortality, Lancet Respir Med, doi:10.1016/S2213-2600(20)30354-4
Ren, Luo, Yu, Song, Liang et al., A randomized, open-label, controlled clinical trial of AZVUDINE tablets in the treatment of mild and common COVID-19, a pilot study, Adv Sci, doi:10.1002/advs.202001435
Wang, Kang, Liu, Tong, Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak, J Med Virol, doi:10.1002/jmv.25721
Winichakoon, Chaiwarith, Liwsrisakun, Salee, Goonna et al., Negative nasopharyngeal and oropharyngeal swab does not rule out COVID-19, J Clin Microbiol, doi:10.1128/JCM.00297-20
Wu, Liu, Zhao, Liu, Wang, Clinical characteristics of imported cases of COVID-19 in Jiangsu Province: a multicenter descriptive study, Clin Infect Dis, doi:10.1093/cid/ciaa199
Yu, Yan, Wang, Yang, Wang et al., Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients, Clin Infect Dis, doi:10.1093/cid/ciaa345
Zhang, Horby, Cao, COVID-19 can be called a treatable disease only after we have antivirals, Sci. Bull, doi:10.1016/j.scib.2022.02.011
Zheng, Yu, Feng, Lou, Zou, Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, Januarymarch 2020: retrospective cohort study, BMJ, doi:10.1136/bmj.m1443
Zhu, Zhang, Li, A novel coronavirus patients with pneumonia in China, N Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.3389/fmed.2023.1215916",
"ISSN": [
"2296-858X"
],
"URL": "http://dx.doi.org/10.3389/fmed.2023.1215916",
"abstract": "<jats:sec><jats:title>Background</jats:title><jats:p>In 2019, a highly pathogenic coronavirus named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) surfaced and resulted in the outbreak of coronavirus disease 2019 (COVID-19). With the aim of finding effective drugs to fight against the disease, several trials have been conducted since COVID-19 can only be considered a treatable disease, from a clinical point of view, after the availability of specific and effective antivirals. AZVUDINE (FNC), initially developed for treating HIV, is a potential treatment for COVID-19 as it has the capability to lower the patient’s viral load and promote recovery.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>Volunteers infected with SARS-CoV-2 confirmed by reverse transcription polymerase chain reaction (RT-PCR), with good kidney and liver function, who were not using other antivirals or monoclonal antibodies were eligible. Samples from patients were assessed for viral load every 48 h during treatment using reverse transcription quantitative polymerase chain reaction (RT-qPCR) and droplet digital polymerase chain reaction (ddPCR).</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>The study’s primary outcome measure was the percentage of participants showing an improvement in clinical scores, while the secondary outcome measure was the percentage of participants with a clinical outcome of cure. These measures were used to assess the safety and efficacy of FNC for treating COVID-19. In the analysis of sociodemographic variables, no significant differences were detected between patients in the FNC and the placebo group for race, age group, or sex. The results showed a potential benefit to participants who received FNC during the study, as observed in the shorter hospital stay, shorter negative conversion time of SARS-CoV-2, and a significant reduction in viral load. Furthermore, the reduction in fever and chills were significant at D1, D2, and D3. In this study, a total of 112 adverse events cases were noted, with 105 cases being categorized as non-serious and only 7 cases as serious adverse events.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>The pandemic is not being effectively controlled and is causing multiple waves of infection that require extensive medical resources. However, FNC has demonstrated potential to reduce the treatment duration of moderate COVID-19 cases, thereby saving significant medical resources. This makes FNC a promising candidate for COVID-19 treatment.</jats:p><jats:p><jats:bold>Clinical trial registration</jats:bold>: [<jats:ext-link>clinicaltrials.gov</jats:ext-link>], identifier [NCT04668235].</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fmed.2023.1215916"
],
"author": [
{
"affiliation": [],
"family": "de Souza",
"given": "Sávio Bastos",
"sequence": "first"
},
{
"affiliation": [],
"family": "Cabral",
"given": "Paula Gebe Abreu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Silva",
"given": "Renato Martins",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arruda",
"given": "Raul Ferraz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabral",
"given": "Sheila Passos de Figueiredo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Assis",
"given": "Arícia Leone Evangelista Monteiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Viana Junior",
"given": "Antônio Brazil",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Degrave",
"given": "Wim Maurits Sylvain",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Moreira",
"given": "Aline dos Santos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Silva",
"given": "Cléber Glória",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Junbiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lei",
"given": "Pingsheng",
"sequence": "additional"
}
],
"container-title": "Frontiers in Medicine",
"container-title-short": "Front. Med.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T07:09:43Z",
"timestamp": 1697699383000
},
"deposited": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T07:09:49Z",
"timestamp": 1697699389000
},
"indexed": {
"date-parts": [
[
2023,
10,
20
]
],
"date-time": "2023-10-20T05:35:50Z",
"timestamp": 1697780150409
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
10,
19
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
19
]
],
"date-time": "2023-10-19T00:00:00Z",
"timestamp": 1697673600000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1215916/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
10,
19
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
19
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"article-title": "A novel coronavirus from patients with pneumonia in China, 2019",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "727",
"journal-title": "N Engl J Med",
"key": "ref1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa199",
"article-title": "Clinical characteristics of imported cases of COVID- 19 in Jiangsu Province: a multicenter descriptive study",
"author": "Wu",
"doi-asserted-by": "publisher",
"first-page": "706",
"journal-title": "Clin Infect Dis",
"key": "ref2",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1002/jmv.25721",
"article-title": "Combination of RT-qPCR testing and clinical features for diagnosis of COVID-19 facilitates management of SARS-CoV-2 outbreak",
"author": "Wang",
"doi-asserted-by": "publisher",
"first-page": "538",
"journal-title": "J Med Virol",
"key": "ref3",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1016/j.scib.2022.02.011",
"article-title": "COVID-19 can be called a treatable disease only after we have antivirals",
"author": "Zhang",
"doi-asserted-by": "publisher",
"first-page": "999",
"journal-title": "Sci. Bull",
"key": "ref4",
"volume": "67",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2007764",
"article-title": "Remdesivir for the treatment of Covid-19—final report",
"author": "Beigel",
"doi-asserted-by": "publisher",
"first-page": "1813",
"journal-title": "N Engl J Med",
"key": "ref5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1681/ASN.2020050589",
"article-title": "Remdesivir in patients with acute or chronic kidney disease and COVID-19",
"author": "Adamsick",
"doi-asserted-by": "publisher",
"first-page": "1384",
"journal-title": "J Am Soc Nephrol",
"key": "ref6",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1101/2020.12.10.419242",
"article-title": "The combined treatment of Molnupiravir and Favipiravir results in a marked potentiation of efficacy in a SARS-CoV2 hamster infection model through an in- creased frequency of mutations in the viral genome",
"author": "Abdelnabi",
"doi-asserted-by": "publisher",
"first-page": "2020",
"journal-title": "bioRxiv",
"key": "ref7",
"year": "2021"
},
{
"DOI": "10.1038/nrd4010",
"article-title": "Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases",
"author": "Jordheim",
"doi-asserted-by": "publisher",
"first-page": "447",
"journal-title": "Nat Rev Drug Discov",
"key": "ref8",
"volume": "12",
"year": "2013"
},
{
"DOI": "10.1002/advs.202001435",
"article-title": "A randomized, open-label, controlled clinical trial of AZVUDINE tablets in the treatment of mild and common COVID-19, a pilot study",
"author": "Ren",
"doi-asserted-by": "publisher",
"first-page": "e2001435",
"journal-title": "Adv Sci",
"key": "ref9",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30232-2",
"article-title": "Viral dynamics in mild and severe cases of COVID-19",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "656",
"journal-title": "Lancet Infect Dis",
"key": "ref10",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa851",
"article-title": "Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019",
"author": "Magleby",
"doi-asserted-by": "publisher",
"first-page": "e4197",
"journal-title": "Clin Infect Dis",
"key": "ref11",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1038/s41467-020-19057-5",
"article-title": "SARS-CoV-2 viral load is associated with increased disease severity and mortality",
"author": "Fajnzylber",
"doi-asserted-by": "publisher",
"first-page": "5493",
"journal-title": "Nat Commun",
"key": "ref12",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30354-4",
"article-title": "SARS-CoV-2 viral load predicts COVID-19 mortality",
"author": "Pujadas",
"doi-asserted-by": "publisher",
"first-page": "E70",
"journal-title": "Lancet Respir Med",
"key": "ref13",
"volume": "8",
"year": "2020"
},
{
"key": "ref14",
"volume-title": "Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases",
"year": ""
},
{
"DOI": "10.1002/jmv.21026",
"article-title": "Respiratory syncytial virus infections in hospitalized infants: association between viral load, virus subgroup, and disease severity",
"author": "Fodha",
"doi-asserted-by": "publisher",
"first-page": "1951",
"journal-title": "J Med Virol",
"key": "ref15",
"volume": "79",
"year": "2007"
},
{
"DOI": "10.1136/bmj.m1443",
"article-title": "Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–march 2020: retrospective cohort study",
"author": "Zheng",
"doi-asserted-by": "publisher",
"first-page": "369m1443",
"journal-title": "BMJ",
"key": "ref16",
"volume": "2020",
"year": "2020"
},
{
"DOI": "10.1016/S1473-3099(20)30113-4",
"article-title": "Viral load of SARS-CoV-2 in clinical samples",
"author": "Pan",
"doi-asserted-by": "publisher",
"first-page": "411",
"journal-title": "Lancet Infect Dis",
"key": "ref17",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa345",
"article-title": "Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients",
"author": "Yu",
"doi-asserted-by": "publisher",
"first-page": "793",
"journal-title": "Clin Infect Dis",
"key": "ref18",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.3389/fmed.2023.1143485",
"article-title": "Serial viral load analysis by DDPCR to evaluate FNC efficacy and safety in the treatment of mild cases of COVID-19",
"author": "da Silva",
"doi-asserted-by": "publisher",
"first-page": "1143485",
"journal-title": "Front Med",
"key": "ref19",
"volume": "10",
"year": "2023"
},
{
"DOI": "10.1128/JCM.00297-20",
"article-title": "Negative nasopharyngeal and oropharyngeal swab does not rule out COVID-19",
"author": "Winichakoon",
"doi-asserted-by": "publisher",
"first-page": "e00297-20",
"journal-title": "J Clin Microbiol",
"key": "ref20",
"volume": "58",
"year": "2020"
},
{
"DOI": "10.1128/aac.02290-20",
"article-title": "A valid warning or clinical Lore: an evaluation of safety outcomes of Remdesivir in patients with impaired renal function from a multicenter matched cohort",
"author": "Ackley",
"doi-asserted-by": "publisher",
"first-page": "e02290",
"journal-title": "Antimicrob Agents Chemother",
"key": "ref21",
"volume": "65",
"year": "2020"
},
{
"DOI": "10.1373/clinchem.2014.230227",
"article-title": "Next generation digital PCR measurement of hepatitis B virus copy number in formalin-fixed paraffin-embedded hepatocellular carcinoma tissue",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "290",
"journal-title": "Clin Chem",
"key": "ref22",
"volume": "61",
"year": "2015"
},
{
"DOI": "10.1038/s41586-019-1027-4",
"article-title": "HIV-1 remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation",
"author": "Gupta",
"doi-asserted-by": "publisher",
"first-page": "244",
"journal-title": "Nature",
"key": "ref23",
"volume": "568",
"year": "2019"
},
{
"DOI": "10.1016/j.jiph.2022.07.002",
"article-title": "A comprehensive genomic study, mutation screening, phylogenetic and statistical analysis of SARS-CoV-2 and its variant omicron among different countries",
"author": "Ahmad",
"doi-asserted-by": "publisher",
"first-page": "878",
"journal-title": "J Infect Public Health",
"key": "ref24",
"volume": "15",
"year": "2022"
}
],
"reference-count": 24,
"references-count": 24,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fmed.2023.1215916/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Phase III, randomized, double-blind, placebo-controlled clinical study: a study on the safety and clinical efficacy of AZVUDINE in moderate COVID-19 patients",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "10"
}
