Effect of Tenofovir Alafenamide Fumarate on the outcomes of hospitalized COVID-19 patients: a prospective, block-balanced, open-label, randomized controlled trial
et al., BMC Pharmacology and Toxicology, doi:10.1186/s40360-024-00781-3, IRCT20200422047168N1, Oct 2024
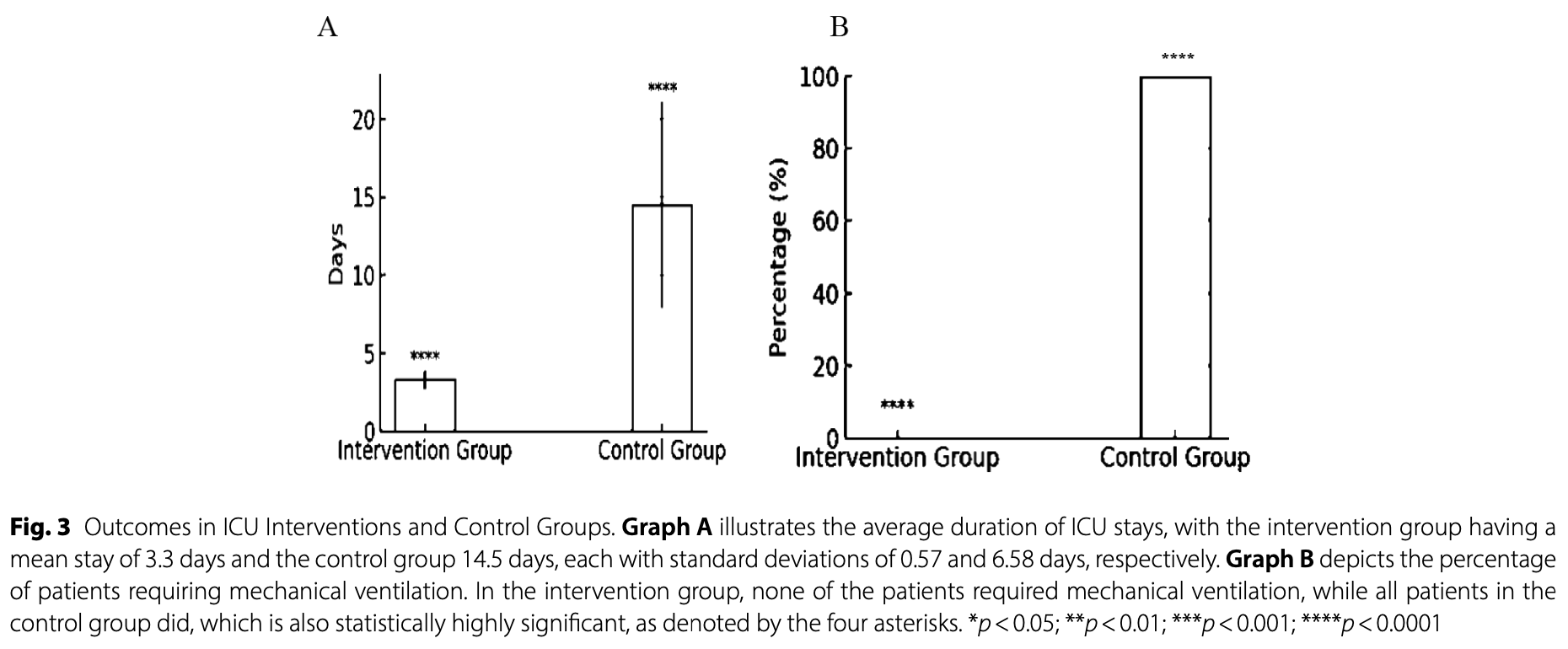
RCT 60 hospitalized COVID-19 patients showing fewer mechanical ventilation and ICU days with tenofovir alafenamide fumarate (TAF) treatment in addition to remdesivir and corticosteroids.
|
risk of mechanical ventilation, 88.9% lower, RR 0.11, p = 0.11, treatment 0 of 30 (0.0%), control 4 of 30 (13.3%), NNT 7.5, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 25.0% lower, RR 0.75, p = 1.00, treatment 3 of 30 (10.0%), control 4 of 30 (13.3%), NNT 30.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Yazdan Pouri et al., 17 Oct 2024, Randomized Controlled Trial, Iran, peer-reviewed, 7 authors, study period September 2020 - February 2021, trial IRCT20200422047168N1.
Contact: zahrashokati@gmail.com, shayeste-a@ajums.ac.ir.
Effect of Tenofovir Alafenamide Fumarate on the outcomes of hospitalized COVID-19 patients: a prospective, block-balanced, open-label, randomized controlled trial
BMC Pharmacology and Toxicology, doi:10.1186/s40360-024-00781-3
Background The global effort to cure COVID-19 is still ongoing. Thus, a prospective, block-balanced, open-label, randomized controlled trial was conducted to evaluate how Tenofovir Alafenamide Fumarate affects hospitalized COVID-19 patients' outcomes.
Methods The intervention and control groups of 60 hospitalized COVID-19 patients were randomly allocated. Along with normal medication, the intervention group received 25 mg of tenofovir orally daily for seven days. The control group got normal therapy, including remdesivir and corticosteroids. ICU hospitalization duration, laboratory data, fever, dyspnea, arterial blood oxygen saturation with and without an oxygen face mask, mechanical ventilation, and mortality were the outcomes.
Results Sixty of 236 eligible patients between September 2020 and February 2021 were enrolled. The intervention group had a mean age (±SD) of 61.33 (±13.09) years and the control group 60.03 (±18.03). Sixteen (53.3%) intervention patients and 15 (50.0%) control patients were males. The intervention group had fewer mechanical ventilation and ICU days. Tenofovir Alafenamide Fumarate did not improve fever, dyspnea, oxygen saturation with or without a face mask or nasal cannula, or laboratory data including WBC, ESR, CRP, AST, ALT, AlkP, total and direct bilirubin, in COVID-19 patients.
Conclusion According to this pilot trial, Tenofovir Alafenamide Fumarate, along with conventional treatment, significantly reduced mechanical ventilation and ICU stay in COVID-19 patients. Further thorough research is necessary to verify this conclusion.
Author contributions N.Y.P. conceived and designed the study. A.T. and F.A. and N.N. performed the intervention and participated in clinical follow-up of the patients. B.C. analyzed and interpreted the data. Z.S.E. and A.A.S. wrote the main manuscript text and managed the study. All authors approved the final version.
Declarations Ethics approval and informed consent This study received ethical approval in 29/04/2020 (IR.AJUMS.REC.1399.082) from the Ahvaz-Jundishapur University of Medical Sciences in Ahvaz, Iran. The protocol was made available on the website WWW.IRCT.ir with the identification IRCT20200422047168N1. Written informed consent was obtained from all the participants, and the study was carried out following the guidelines of the Declaration of Helsinki.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Amo, Polo, Moreno, Díaz, Martínez et al., Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons, Epidemiology
Amo, Polo, Moreno, Díaz, Martínez et al., Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study, Ann Intern Med
Birkus, Bam, Willkom, Frey, Tsai et al., Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors, Antimicrob Agents Chemother
Cascella, Rajnik, Aleem, Dulebohn, Napoli, Features, evaluation, and treatment of coronavirus (COVID-19)
Clercq, Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF), Biochem Pharmacol
Clososki, Soldi, Rmd, Guaratini, Lopes et al., Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2, J Braz Chem Soc
Davis, Ferreira, Denholm, Tong, Clinical trials for the prevention and treatment of COVID-19: current state of play, Med J Aust
Dejong, Spinelli, Okochi, Gandhi, Tenofovir-based PrEP for COVID-19: an untapped opportunity?, AIDS
Drosu, Edelman, Housman, Tenofovir prodrugs potently inhibit Epstein-Barr virus lytic DNA replication by targeting the viral DNA polymerase, Proc Natl Acad Sci
Eisenberg, He, Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood, Nucleosides Nucleotides Nucleic Acids
Eshkiki, Shahriari, Seyedtabib, Torabizadeh, Assarehzadegan et al., Innate and adaptive immunity imbalance with severe COVID-19 pneumonia in children and adults, Front Pediatr
García-Albéniz, Amo, Polo, Morales-Asencio, Hernán, Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19, Eur J Epidemiol
Guan, Zhong, Clinical characteristics of Covid-19 in China, N Engl J Med
Hernandez-Diaz, Bateman, Straub, Zhu, Mogun et al., Safety of tenofovir disoproxil fumarate for pregnant women facing the coronavirus disease 2019 pandemic, Am J Epidemiol
Huang, Wang, Ye, Da, Fang et al., Dynamic changes of T-lymphocyte subsets and the correlations with 89 patients with coronavirus disease 2019 (COVID-19), Ann Transl Med
Jr, Lima, Duarte, Powell, Ormsby et al., Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection, J Biomol Struct Dyn
Kow, Ramachandram, Hasan, The use of tenofovir in patients with COVID-19, HIV Med
Levitan, Pulse oximetry as a biomarker for early identification and hospitalization of COVID-19 pneumonia, Acad Emerg Med
Lorizate, Kräusslich, Role of lipids in virus replication, Cold Spring Harb Perspect Biol
Markowitz, Zolopa, Ruane, Squires, Zhong et al., GS-7340 demonstrates greater declines in HIV-1 RNA than tenofovir disoproxil fumarate during 14 days of monotherapy in HIV-1 infected subjects
Mateos-Muñoz, Buti, Vázquez, Conde, Bernal-Monterde et al., Tenofovir disoproxil fumarate reduces the severity of COVID-19 in patients with chronic hepatitis B, Dig Dis Sci
Melchjorsen, Risør, Søgaard, 'loughlin, Chow et al., Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells, J Acquir Immune Defic Syndr
Mitjà, Corbacho-Monné, Ubals, Tebé, Peñafiel et al., Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial, Clin Infect Dis
Naggie, Milstone, Castro, Collins, Lakshmi et al., Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial Healthcare Worker exposure response and outcomes of Hydroxychloroquine (HERO-HCQ), Int J Infect Dis
Painter, Sheahan, Baric, Holman, Donovan et al., Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with molnupiravir, Top Antivir Med
Patterson, Prince, Kraft, Jenkins, Shaheen et al., Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission, Sci Transl Med
Peluso, Abella, Ferrer, Kucher, Sunde et al., Fever management in COVID-19 patients, Minerva Anestesiol
Polo, García-Albéniz, Terán, Morales, Rial-Crestelo et al., Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebocontrolled randomized trial in healthcare workers, Clin Microbiol Infect
Pramesh, Babu, Basu, Bhushan, Booth et al., Choosing wisely for COVID-19: ten evidence-based recommendations for patients and physicians, Nat Med
Rommasi, Nasiri, Mirsaiedi, Antiviral drugs proposed for COVID-19: action mechanism and pharmacological data, Eur Rev Med Pharmacol Sci
Seifert, Chen, Meditz, Castillo-Mancilla, Gardner et al., Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state, AIDS Res Hum Retroviruses
Skipper, Pastick, Engen, Bangdiwala, Abassi et al., Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial, Ann Inter Med
Zanella, Zizioli, Castelli, Quiros-Roldan, Tenofovir, another inexpensive, well-known and widely available old drug repurposed for SARS-COV-2 infection, Pharmaceuticals
Zk, Franková, Holý, Activation by 9®[2-(phosphonomethoxy) propyl] adenine of chemokine (RANTES, macrophage inflammatory protein 1α) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1β) production, Antimicrob Agents Chemother
DOI record:
{
"DOI": "10.1186/s40360-024-00781-3",
"ISSN": [
"2050-6511"
],
"URL": "http://dx.doi.org/10.1186/s40360-024-00781-3",
"alternative-id": [
"781"
],
"article-number": "78",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "8 June 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 August 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "17 October 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and informed consent",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This study received ethical approval in 29/04/2020 (IR.AJUMS.REC.1399.082) from the Ahvaz-Jundishapur University of Medical Sciences in Ahvaz, Iran. The protocol was made available on the website with the identification IRCT20200422047168N1. Written informed consent was obtained from all the participants, and the study was carried out following the guidelines of the Declaration of Helsinki."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Yazdan Pouri",
"given": "Nazanin",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shokati Eshkiki",
"given": "Zahra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Talebi",
"given": "Afshin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheraghian",
"given": "Bahman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ahmadi",
"given": "Fatemeh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Neisi",
"given": "Niloofar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Shayesteh",
"given": "Ali Akbar",
"sequence": "additional"
}
],
"container-title": "BMC Pharmacology and Toxicology",
"container-title-short": "BMC Pharmacol Toxicol",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T09:02:37Z",
"timestamp": 1729155757000
},
"deposited": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T19:05:23Z",
"timestamp": 1729191923000
},
"indexed": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T19:40:24Z",
"timestamp": 1729194024999,
"version": "3.27.0"
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
10,
17
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T00:00:00Z",
"timestamp": 1729123200000
}
},
{
"URL": "https://creativecommons.org/licenses/by-nc-nd/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
10,
17
]
],
"date-time": "2024-10-17T00:00:00Z",
"timestamp": 1729123200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40360-024-00781-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40360-024-00781-3/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40360-024-00781-3.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
10,
17
]
]
},
"published-online": {
"date-parts": [
[
2024,
10,
17
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.5694/mja2.50673",
"author": "JS Davis",
"doi-asserted-by": "publisher",
"first-page": "86",
"issue": "2",
"journal-title": "Med J Aust",
"key": "781_CR1",
"unstructured": "Davis JS, Ferreira D, Denholm JT, Tong SY. Clinical trials for the prevention and treatment of COVID-19: current state of play. Med J Aust. 2020;213(2):86–93.",
"volume": "213",
"year": "2020"
},
{
"author": "F Rommasi",
"first-page": "4163",
"issue": "11",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "781_CR2",
"unstructured": "Rommasi F, Nasiri MJ, Mirsaiedi M. Antiviral drugs proposed for COVID-19: action mechanism and pharmacological data. Eur Rev Med Pharmacol Sci. 2021;25(11):4163–73.",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01439-x",
"author": "CS Pramesh",
"doi-asserted-by": "publisher",
"first-page": "1324",
"issue": "8",
"journal-title": "Nat Med",
"key": "781_CR3",
"unstructured": "Pramesh CS, Babu GR, Basu J, Bhushan I, Booth CM, Chinnaswamy G, et al. Choosing wisely for COVID-19: ten evidence-based recommendations for patients and physicians. Nat Med. 2021;27(8):1324–7.",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.1007/s10654-022-00891-4",
"author": "X García-Albéniz",
"doi-asserted-by": "publisher",
"first-page": "789",
"issue": "8",
"journal-title": "Eur J Epidemiol",
"key": "781_CR4",
"unstructured": "García-Albéniz X, Del Amo J, Polo R, Morales-Asencio JM, Hernán MA. Systematic review and meta-analysis of randomized trials of hydroxychloroquine for the prevention of COVID-19. Eur J Epidemiol. 2022;37(8):789–96.",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.1016/j.ijid.2023.01.019",
"author": "S Naggie",
"doi-asserted-by": "publisher",
"first-page": "40",
"journal-title": "Int J Infect Dis",
"key": "781_CR5",
"unstructured": "Naggie S, Milstone A, Castro M, Collins SP, Lakshmi S, Anderson DJ, et al. Hydroxychloroquine for pre-exposure prophylaxis of COVID-19 in health care workers: a randomized, multicenter, placebo-controlled trial Healthcare Worker exposure response and outcomes of Hydroxychloroquine (HERO-HCQ). Int J Infect Dis. 2023;129:40–8.",
"volume": "129",
"year": "2023"
},
{
"DOI": "10.7326/M20-3689",
"author": "J Del Amo",
"doi-asserted-by": "publisher",
"first-page": "536",
"issue": "7",
"journal-title": "Ann Intern Med",
"key": "781_CR6",
"unstructured": "Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med. 2020;173(7):536–41.",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1097/EDE.0000000000001235",
"author": "J Del Amo",
"doi-asserted-by": "publisher",
"first-page": "e49",
"issue": "6",
"journal-title": "Epidemiology",
"key": "781_CR7",
"unstructured": "Del Amo J, Polo R, Moreno S, Díaz A, Martínez E, Arribas JR, et al. Antiretrovirals and risk of COVID-19 diagnosis and hospitalization in HIV-positive persons. Epidemiology. 2020;31(6):e49–51.",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1080/07391102.2021.1901144",
"author": "DC Copertino Jr",
"doi-asserted-by": "publisher",
"first-page": "7367",
"issue": "16",
"journal-title": "J Biomol Struct Dyn",
"key": "781_CR8",
"unstructured": "Copertino Jr DC, Casado Lima BC, Duarte RR, Powell TR, Ormsby CE, Wilkin T, et al. Antiretroviral drug activity and potential for pre-exposure prophylaxis against COVID-19 and HIV infection. J Biomol Struct Dyn. 2022;40(16):7367–80.",
"volume": "40",
"year": "2022"
},
{
"DOI": "10.3390/ph14050454",
"author": "I Zanella",
"doi-asserted-by": "publisher",
"first-page": "454",
"issue": "5",
"journal-title": "Pharmaceuticals",
"key": "781_CR9",
"unstructured": "Zanella I, Zizioli D, Castelli F, Quiros-Roldan E. Tenofovir, another inexpensive, well-known and widely available old drug repurposed for SARS-COV-2 infection. Pharmaceuticals. 2021;14(5):454.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1089/aid.2016.0008",
"author": "SM Seifert",
"doi-asserted-by": "publisher",
"first-page": "981",
"issue": "10–11",
"journal-title": "AIDS Res Hum Retroviruses",
"key": "781_CR10",
"unstructured": "Seifert SM, Chen X, Meditz AL, Castillo-Mancilla JR, Gardner EM, Predhomme JA, et al. Intracellular tenofovir and emtricitabine anabolites in genital, rectal, and blood compartments from first dose to steady state. AIDS Res Hum Retroviruses. 2016;32(10–11):981–91.",
"volume": "32",
"year": "2016"
},
{
"DOI": "10.1126/scitranslmed.3003174",
"author": "KB Patterson",
"doi-asserted-by": "publisher",
"first-page": "re1124",
"issue": "112",
"journal-title": "Sci Transl Med",
"key": "781_CR11",
"unstructured": "Patterson KB, Prince HA, Kraft E, Jenkins AJ, Shaheen NJ, Rooney JF, et al. Penetration of tenofovir and emtricitabine in mucosal tissues: implications for prevention of HIV-1 transmission. Sci Transl Med. 2011;3(112):re1124–4.",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1016/j.bcp.2016.04.015",
"author": "E De Clercq",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Biochem Pharmacol",
"key": "781_CR12",
"unstructured": "De Clercq E. Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF). Biochem Pharmacol. 2016;119:1–7.",
"volume": "119",
"year": "2016"
},
{
"DOI": "10.1073/pnas.2002392117",
"author": "NC Drosu",
"doi-asserted-by": "publisher",
"first-page": "12368",
"issue": "22",
"journal-title": "Proc Natl Acad Sci",
"key": "781_CR13",
"unstructured": "Drosu NC, Edelman ER, Housman DE. Tenofovir prodrugs potently inhibit Epstein–Barr virus lytic DNA replication by targeting the viral DNA polymerase. Proc Natl Acad Sci. 2020;117(22):12368–74.",
"volume": "117",
"year": "2020"
},
{
"author": "GC Clososki",
"first-page": "1552",
"journal-title": "J Braz Chem Soc",
"key": "781_CR14",
"unstructured": "Clososki GC, Soldi RA, Silva RMd, Guaratini T, Lopes JN, Pereira PR, et al. Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2. J Braz Chem Soc. 2020;31:1552–6.",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01834-15",
"author": "G Birkus",
"doi-asserted-by": "publisher",
"first-page": "316",
"issue": "1",
"journal-title": "Antimicrob Agents Chemother",
"key": "781_CR15",
"unstructured": "Birkus G, Bam RA, Willkom M, Frey CR, Tsai L, Stray KM, et al. Intracellular activation of tenofovir alafenamide and the effect of viral and host protease inhibitors. Antimicrob Agents Chemother. 2016;60(1):316–22.",
"volume": "60",
"year": "2016"
},
{
"DOI": "10.21037/atm-20-5479",
"doi-asserted-by": "crossref",
"key": "781_CR16",
"unstructured": "Huang M, Wang Y, Ye J, Da H, Fang S, Chen L. Dynamic changes of T-lymphocyte subsets and the correlations with 89 patients with coronavirus disease 2019 (COVID-19). Ann Transl Med. 2020;8(18)."
},
{
"DOI": "10.1093/cid/ciaa1009",
"author": "O Mitjà",
"doi-asserted-by": "publisher",
"first-page": "e4073",
"issue": "11",
"journal-title": "Clin Infect Dis",
"key": "781_CR17",
"unstructured": "Mitjà O, Corbacho-Monné M, Ubals M, Tebé C, Peñafiel J, Tobias A, et al. Hydroxychloroquine for early treatment of adults with mild coronavirus disease 2019: a randomized, controlled trial. Clin Infect Dis. 2021;73(11):e4073–81.",
"volume": "73",
"year": "2021"
},
{
"DOI": "10.7326/M20-4207",
"author": "CP Skipper",
"doi-asserted-by": "publisher",
"first-page": "623",
"issue": "8",
"journal-title": "Ann Inter Med",
"key": "781_CR18",
"unstructured": "Skipper CP, Pastick KA, Engen NW, Bangdiwala AS, Abassi M, Lofgren SM, et al. Hydroxychloroquine in nonhospitalized adults with early COVID-19: a randomized trial. Ann Inter Med. 2020;173(8):623–31.",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1101/cshperspect.a004820",
"author": "M Lorizate",
"doi-asserted-by": "publisher",
"first-page": "a004820",
"issue": "10",
"journal-title": "Cold Spring Harb Perspect Biol",
"key": "781_CR19",
"unstructured": "Lorizate M, Kräusslich H-G. Role of lipids in virus replication. Cold Spring Harb Perspect Biol. 2011;3(10):a004820.",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1097/QAI.0b013e3182185276",
"author": "J Melchjorsen",
"doi-asserted-by": "publisher",
"first-page": "265",
"issue": "4",
"journal-title": "J Acquir Immune Defic Syndr",
"key": "781_CR20",
"unstructured": "Melchjorsen J, Risør MW, Søgaard OS, O’Loughlin KL, Chow S, Paludan SR, et al. Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells. J Acquir Immune Defic Syndr. 2011;57(4):265–75.",
"volume": "57",
"year": "2011"
},
{
"DOI": "10.1128/AAC.45.12.3381-3386.2001",
"author": "D Zı́dek Zk, Franková",
"doi-asserted-by": "publisher",
"first-page": "3381",
"issue": "12",
"journal-title": "Antimicrob Agents Chemother",
"key": "781_CR21",
"unstructured": "Zı́dek Zk, Franková D, Holý A. Activation by 9®[2-(phosphonomethoxy) propyl] adenine of chemokine (RANTES, macrophage inflammatory protein 1α) and cytokine (tumor necrosis factor alpha, interleukin-10 [IL-10], IL-1β) production. Antimicrob Agents Chemother. 2001;45(12):3381–6.",
"volume": "45",
"year": "2001"
},
{
"DOI": "10.1081/NCN-100002496",
"author": "EJ Eisenberg",
"doi-asserted-by": "publisher",
"first-page": "1091",
"issue": "4–7",
"journal-title": "Nucleosides Nucleotides Nucleic Acids",
"key": "781_CR22",
"unstructured": "Eisenberg EJ, He G-X, Lee WA. Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood. Nucleosides Nucleotides Nucleic Acids. 2001;20(4–7):1091–8.",
"volume": "20",
"year": "2001"
},
{
"key": "781_CR23",
"unstructured": "Markowitz M, Zolopa A, Ruane P, Squires K, Zhong L, Kearney B, et al. editors. GS-7340 demonstrates greater declines in HIV-1 RNA than tenofovir disoproxil fumarate during 14 days of monotherapy in HIV-1 infected subjects. 18th Conference on Retroviruses and Opportunistic Infections; 2011."
},
{
"author": "WJ Guan",
"first-page": "1861",
"issue": "19",
"journal-title": "N Engl J Med",
"key": "781_CR24",
"unstructured": "Guan WJ, Zhong NS. Clinical characteristics of Covid-19 in China. Reply. N Engl J Med. 2020;382(19):1861–2.",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.23736/S0375-9393.20.15195-2",
"author": "L Peluso",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Minerva Anestesiol",
"key": "781_CR25",
"unstructured": "Peluso L, Abella BS, Ferrer R, Kucher N, Sunde K, Taccone FS. Fever management in COVID-19 patients. Minerva Anestesiol. 2021;87(1):1–3.",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.3389/fped.2021.736013",
"author": "Z Shokati Eshkiki",
"doi-asserted-by": "publisher",
"first-page": "736013",
"journal-title": "Front Pediatr",
"key": "781_CR26",
"unstructured": "Shokati Eshkiki Z, Shahriari A, Seyedtabib M, Torabizadeh M, Assarehzadegan MA, Nashibi R, Khosravi M, Neisi N, Mard SA, Shayesteh AA. Innate and adaptive immunity imbalance with severe COVID-19 pneumonia in children and adults. Front Pediatr. 2021;9:736013.",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1111/acem.14052",
"author": "RM Levitan",
"doi-asserted-by": "publisher",
"first-page": "785",
"issue": "8",
"journal-title": "Acad Emerg Med",
"key": "781_CR27",
"unstructured": "Levitan RM. Pulse oximetry as a biomarker for early identification and hospitalization of COVID-19 pneumonia. Acad Emerg Med. 2020;27(8):785.",
"volume": "27",
"year": "2020"
},
{
"key": "781_CR28",
"unstructured": "Cascella M, Rajnik M, Aleem A, Dulebohn SC, Di Napoli R. Features, evaluation, and treatment of coronavirus (COVID-19). Statpearls [internet]. 2022."
},
{
"DOI": "10.1111/hiv.13228",
"author": "CS Kow",
"doi-asserted-by": "publisher",
"first-page": "807",
"issue": "7",
"journal-title": "HIV Med",
"key": "781_CR29",
"unstructured": "Kow CS, Ramachandram DS, Hasan SS. The use of tenofovir in patients with COVID-19. HIV Med. 2022;23(7):807–8.",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.1007/s10620-022-07817-w",
"doi-asserted-by": "crossref",
"key": "781_CR30",
"unstructured": "Mateos-Muñoz B, Buti M, Vázquez IF, Conde MH, Bernal-Monterde V, Díaz-Fontenla F, et al. Tenofovir disoproxil fumarate reduces the severity of COVID-19 in patients with chronic hepatitis B. Dig Dis Sci. 2023:1–7."
},
{
"DOI": "10.1016/j.cmi.2022.07.006",
"author": "R Polo",
"doi-asserted-by": "publisher",
"first-page": "85",
"issue": "1",
"journal-title": "Clin Microbiol Infect",
"key": "781_CR31",
"unstructured": "Polo R, García-Albéniz X, Terán C, Morales M, Rial-Crestelo D, Garcinuño MA, et al. Daily tenofovir disoproxil fumarate/emtricitabine and hydroxychloroquine for pre-exposure prophylaxis of COVID-19: a double-blind placebo-controlled randomized trial in healthcare workers. Clin Microbiol Infect. 2023;29(1):85–93.",
"volume": "29",
"year": "2023"
},
{
"key": "781_CR32",
"unstructured": "Painter WP, Sheahan T, Baric R, Holman W, Donovan J, Fang L, et al. Reduction in infectious SARS-CoV-2 in treatment study of COVID-19 with molnupiravir. Top Antivir Med. 2021:304–5."
},
{
"DOI": "10.1097/QAD.0000000000002877",
"author": "C DeJong",
"doi-asserted-by": "publisher",
"first-page": "1509",
"issue": "9",
"journal-title": "AIDS",
"key": "781_CR33",
"unstructured": "DeJong C, Spinelli MA, Okochi H, Gandhi M. Tenofovir-based PrEP for COVID-19: an untapped opportunity? AIDS. 2021;35(9):1509.",
"volume": "35",
"year": "2021"
},
{
"DOI": "10.1093/aje/kwab109",
"author": "S Hernandez-Diaz",
"doi-asserted-by": "publisher",
"first-page": "2339",
"issue": "11",
"journal-title": "Am J Epidemiol",
"key": "781_CR34",
"unstructured": "Hernandez-Diaz S, Bateman BT, Straub L, Zhu Y, Mogun H, Fischer M, et al. Safety of tenofovir disoproxil fumarate for pregnant women facing the coronavirus disease 2019 pandemic. Am J Epidemiol. 2021;190(11):2339–49.",
"volume": "190",
"year": "2021"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcpharmacoltoxicol.biomedcentral.com/articles/10.1186/s40360-024-00781-3"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effect of Tenofovir Alafenamide Fumarate on the outcomes of hospitalized COVID-19 patients: a prospective, block-balanced, open-label, randomized controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "25"
}