Reduced Viral Shedding Time in High-Risk COVID-19 Patients Infected by Omicron and Treated with Paxlovid: A Real-World Study from China
et al., Infection and Drug Resistance, doi:10.2147/IDR.S443574, Mar 2024
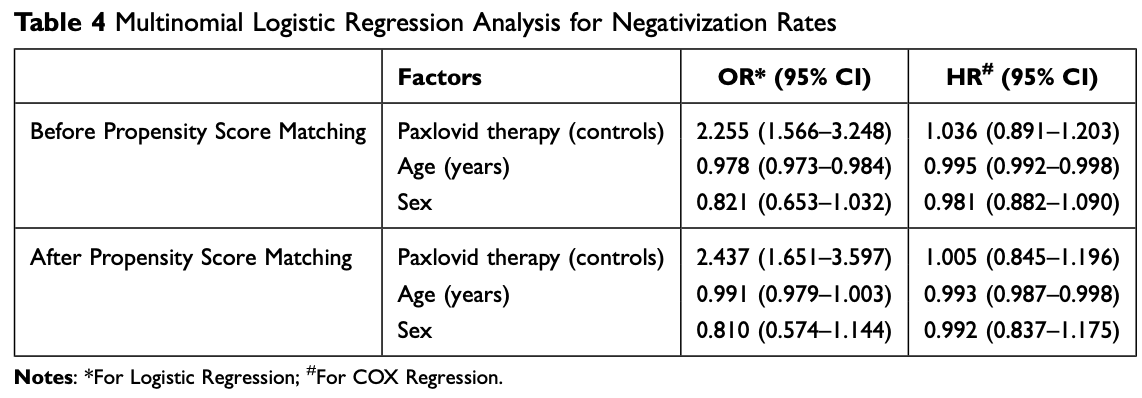
Retrospective 3,159 high risk COVID-19 patients in China showing no significant difference for viral clearance with multivariable Cox regression, but significantly faster viral clearance with logistic regression. Cox results account for the time to events and can handle censored data, and may be more clinically meaningful because they directly relate to the instantaneous risk of an event occurring. Authors do not discuss the large difference between the Cox and logistic regression results, which may bre related to increased rebound with paxlovid, time-dependent treatment effects, baseline differences, censored data, and unmeasured confounding.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
|
risk of no viral clearance, 0.5% lower, HR 1.00, p = 0.96, treatment 362, control 724, adjusted per study, inverted to make HR<1 favor treatment, multivariable, Cox proportional hazards.
|
|
risk of no viral clearance, 59.0% lower, OR 0.41, p < 0.001, treatment 362, control 724, adjusted per study, inverted to make OR<1 favor treatment, logistic regression, multivariable, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Yang et al., 29 Mar 2024, retrospective, China, peer-reviewed, 10 authors, study period March 2022 - April 2022.
Contact: drkaijiang@163.com, mingyan1970@126.com.
Reduced Viral Shedding Time in High-Risk COVID-19 Patients Infected by Omicron and Treated with Paxlovid: A Real-World Study from China
Infection and Drug Resistance, doi:10.2147/idr.s443574
Introduction: The purpose of this study was to compare the viral shedding time in patients infected with the Omicron variant during Paxlovid therapy and conventional therapy and to analyze the effects of Paxlovid on patients infected with COVID-19. Methods: In this study, the demographic and clinical characteristics and laboratory data of 3159 patients infected with the SARS-CoV -2 Omicron variant treated at Jilin Province People's Hospital were collected and analyzed. A total of 362 patients received Paxlovid therapy, and 2797 patients received conventional therapy. After propensity score matching (PSM), 1086 patients were obtained.
Results: The difference in platelet (PLT) count between the two groups was statistically significant but within the normal range (P < 0.05). CT value revealed that the nucleic acid test results became negative more quickly in the Paxlovid therapy group. Analysis of the Paxlovid therapy group showed that IgG and IgM levels were increased after Paxlovid therapy administration.
Conclusion: The CT value of the Paxlovid therapy group became negative more quickly. This finding suggests that Paxlovid treatment after early diagnosis of the Omicron variant may achieve good therapeutic efficacy.
Conclusions Our data show that the CT value of the Paxlovid therapy group became negative faster than that of the control group, suggesting that the use of the Paxlovid in Omicron variant may achieve excellent therapeutic efficacy after early diagnosis. Moreover, Paxlovid should be used with caution and only in high-risk patients.
Author Contributions All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure The authors declare that they have no conflicts of interest in this work.
References
Adam, What scientists know about new, fast-spreading coronavirus variants, Nature, doi:10.1038/d41586-021-01390-4
Cegolon, Magnano, Negro, Filon, SARS-CoV-2 reinfections in health-care workers, 1 March 2020-31, Viruses, doi:10.3390/v15071551
Cegolon, Negro, Mastrangelo, Filon, Primary SARS-CoV-2 infections, re-infections and vaccine effectiveness during the Omicron transmission period in healthcare workers of Trieste and Gorizia (Northeast Italy), Viruses, doi:10.3390/v14122688
Cegolon, Pol, Simonetti, Filon, Luzzati, Molnupiravir, nirmatrelvir/ritonavir, or sotrovimab for high-risk COVID-19 patients infected by the Omicron variant: hospitalization, mortality, and time until negative swab test in real life, Pharmaceuticals, doi:10.3390/ph16050721
Chen, Gao, Wang, Wei, Prediction and mitigation of mutation threats to COVID-19 vaccines and antibody therapies, Chem. Sci, doi:10.1039/D1SC01203G
Hammond, Leister-Tebbe, Gardner, Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19, New Engl J Med, doi:10.1056/NEJMoa2118542
Li, Guan, Wu, Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia, New Engl J Med, doi:10.1056/NEJMoa2001316
Li, Li, Li, Zhang, Ma, Clinical characteristics and analysis of factors associated with severe COVID-19 patients in Liaoning, China: a multicenter retrospective study, J Transl Crit Care Med, doi:10.4103/jtccm.jtccm_7_21
Liu, Rocklöv, The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus, J Travel Med, doi:10.1093/jtm/taab124
Long, Carius, Chavez, Clinical update on COVID-19 for the emergency clinician: presentation and evaluation, Am J Emergency Med, doi:10.1016/j.ajem.2022.01.028
Mahase, Covid-19: UK becomes first country to authorise antiviral molnupiravir, BMJ
Mahase, Covid-19: pfizer's paxlovid is 89% effective in patients at risk of serious illness, company reports, and Drug Resistance, doi:10.2147/IDR.S443574DovePressInfection
Owen, Allerton, Anderson, An oral SARS-CoV-2 M(pro) inhibitor clinical candidate for the treatment of COVID-19, Science, doi:10.1126/science.abl4784
Viana, Moyo, Amoako, Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in Southern Africa, Nature, doi:10.1038/s41586-022-04411-y
Vito, Moi, Saderi, Vaccination and antiviral treatment reduce the time to negative SARS-CoV-2 Swab: a real-life study, doi:10.3390/v15112180
Zhu, Zhang, Wang, A novel coronavirus from patients with pneumonia in China, 2019, New Engl J Med, doi:10.1056/NEJMoa2001017
DOI record:
{
"DOI": "10.2147/idr.s443574",
"ISSN": [
"1178-6973"
],
"URL": "http://dx.doi.org/10.2147/IDR.S443574",
"author": [
{
"affiliation": [],
"family": "Yang",
"given": "Wei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Peng",
"given": "Yahui",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-0079-5259",
"affiliation": [],
"authenticated-orcid": true,
"family": "Wang",
"given": "Changsong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cai",
"given": "Hongliu",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Lina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xu",
"given": "Jun",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yongjie",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Maonan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhao",
"given": "Mingyan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yu",
"given": "Kaijiang",
"sequence": "additional"
}
],
"container-title": "Infection and Drug Resistance",
"container-title-short": "IDR",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T07:25:09Z",
"timestamp": 1711697109000
},
"deposited": {
"date-parts": [
[
2024,
3,
29
]
],
"date-time": "2024-03-29T07:25:14Z",
"timestamp": 1711697114000
},
"indexed": {
"date-parts": [
[
2024,
3,
30
]
],
"date-time": "2024-03-30T01:17:47Z",
"timestamp": 1711761467663
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2024,
3
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by-nc/3.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
1
]
],
"date-time": "2024-03-01T00:00:00Z",
"timestamp": 1709251200000
}
}
],
"link": [
{
"URL": "https://www.dovepress.com/getfile.php?fileID=97994",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.dovepress.com/getfile.php?fileID=97994",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "1267-1279",
"prefix": "10.2147",
"published": {
"date-parts": [
[
2024,
3
]
]
},
"published-online": {
"date-parts": [
[
2024,
3
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"DOI": "10.1056/NEJMoa2001017",
"author": "Zhu",
"doi-asserted-by": "publisher",
"first-page": "727",
"journal-title": "New Engl J Med",
"key": "ref1",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2001316",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "1199",
"journal-title": "New Engl J Med",
"key": "ref2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.4103/jtccm.jtccm_7_21",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "90",
"journal-title": "J Transl Crit Care Med",
"key": "ref3",
"volume": "2",
"year": "2020"
},
{
"key": "ref4",
"unstructured": "World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19; 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-The-media-briefing-on-covid-19---11-march-2020. Accessed March 11, 2020."
},
{
"key": "ref5",
"unstructured": "World Health Organization. Tracking SARS-CoV-2 variants. Available from: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants. Accessed February 21, 2022."
},
{
"DOI": "10.1038/s41586-022-04411-y",
"author": "Viana",
"doi-asserted-by": "publisher",
"first-page": "679",
"journal-title": "Nature",
"key": "ref6",
"volume": "603",
"year": "2022"
},
{
"DOI": "10.1038/d41586-021-01390-4",
"author": "Adam",
"doi-asserted-by": "publisher",
"first-page": "19",
"journal-title": "Nature",
"key": "ref7",
"volume": "594",
"year": "2021"
},
{
"key": "ref8",
"unstructured": "Centers for Disease Control and Prevention. COVID data tracker: variant pro-portions; 2022. Available from: https://covid.cdc.gov/covid-data-tracker/#variant-proportions. Accessed March 25, 2024."
},
{
"DOI": "10.1039/D1SC01203G",
"author": "Chen",
"doi-asserted-by": "publisher",
"first-page": "6929",
"journal-title": "Chem. Sci.",
"key": "ref9",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1093/jtm/taab124",
"author": "Liu",
"doi-asserted-by": "publisher",
"journal-title": "J Travel Med",
"key": "ref10",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1016/j.ajem.2022.01.028",
"author": "Long",
"doi-asserted-by": "publisher",
"first-page": "46",
"journal-title": "Am J Emergency Med",
"key": "ref11",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.3390/v15071551",
"author": "Cegolon",
"doi-asserted-by": "publisher",
"first-page": "1551",
"journal-title": "Viruses",
"key": "ref12",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3390/v14122688",
"author": "Cegolon",
"doi-asserted-by": "publisher",
"first-page": "2688",
"journal-title": "Viruses",
"key": "ref13",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1126/science.abl4784",
"author": "Owen",
"doi-asserted-by": "publisher",
"first-page": "1586",
"journal-title": "Science",
"key": "ref14",
"volume": "374",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2697",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "n2697",
"journal-title": "BMJ",
"key": "ref15",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.1136/bmj.n2713",
"author": "Mahase",
"doi-asserted-by": "crossref",
"first-page": "n2713",
"journal-title": "BMJ",
"key": "ref16",
"volume": "375",
"year": "2021"
},
{
"DOI": "10.3390/ph16050721",
"author": "Cegolon",
"doi-asserted-by": "publisher",
"first-page": "721",
"journal-title": "Pharmaceuticals",
"key": "ref17",
"volume": "16",
"year": "2023"
},
{
"DOI": "10.1056/NEJMoa2118542",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "New Engl J Med",
"key": "ref18",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.3390/v15112180",
"author": "De Vito",
"doi-asserted-by": "publisher",
"first-page": "2180",
"journal-title": "Viruses",
"key": "ref19",
"volume": "15",
"year": "2023"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.dovepress.com/reduced-viral-shedding-time-in-high-risk-covid-19-patients-infected-by-peer-reviewed-fulltext-article-IDR"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pharmacology (medical)",
"Infectious Diseases",
"Pharmacology"
],
"subtitle": [],
"title": "Reduced Viral Shedding Time in High-Risk COVID-19 Patients Infected by Omicron and Treated with Paxlovid: A Real-World Study from China",
"type": "journal-article",
"volume": "Volume 17"
}