The efficacy and safety of inhaled peptide YKYY017 for COVID-19 patients with mild illness: a phase 2 randomized controlled trial
et al., Nature Communication, doi:0.1038/s41467-025-62214-x, ChiCTR2300075467, Aug 2025
RCT 239 mostly mild COVID-19 outpatients showing the primary endpoint of viral load reduction at day 4 was not met with inhaled peptide YKYY017. The 20mg group showed significantly faster time to recovery. The study population was low-risk, with only one patient progressing to severe disease.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments1.
|
risk of progression, 150.3% higher, RR 2.50, p = 1.00, treatment 1 of 159 (0.6%), control 0 of 80 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
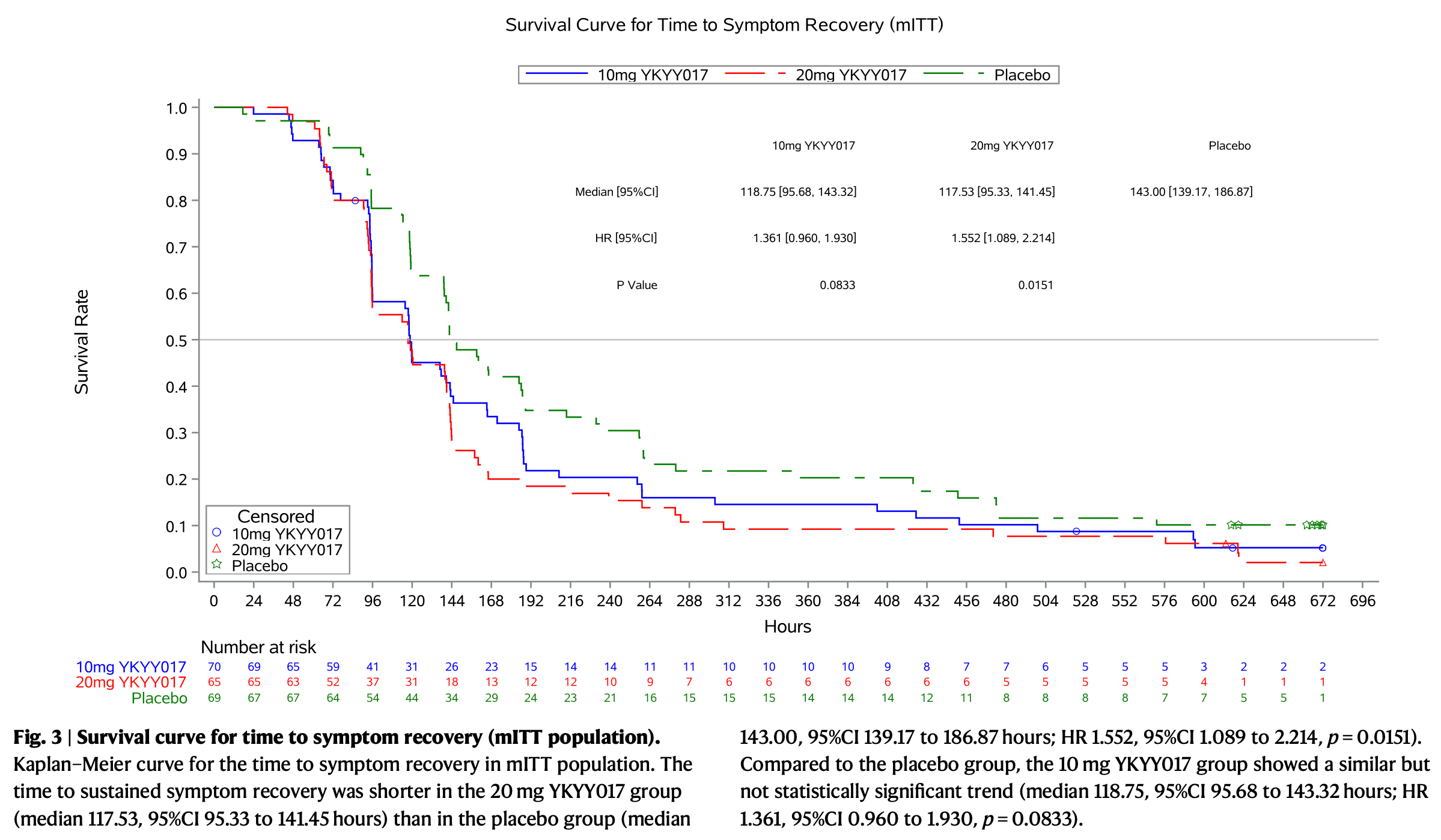
risk of no recovery, 31.1% lower, HR 0.69, p = 0.003, treatment 70, control 69, all patients.
|
|
risk of no recovery, 35.6% lower, HR 0.64, p = 0.02, treatment 65, control 69, inverted to make HR<1 favor treatment, 20mg.
|
|
risk of no recovery, 26.5% lower, HR 0.73, p = 0.08, treatment 70, control 69, inverted to make HR<1 favor treatment, 10mg.
|
|
risk of viral load, 12.5% lower, RR 0.87, p = 0.08, treatment 70, control 69, all patients.
|
|
risk of viral load, 15.9% lower, RR 0.84, p = 0.12, treatment mean 3.02 (±1.93) n=65, control mean 2.54 (±1.66) n=69, 20mg, relative viral load reduction, mid-recovery, day 4.
|
|
risk of viral load, 9.6% lower, RR 0.90, p = 0.33, treatment mean 2.81 (±1.63) n=70, control mean 2.54 (±1.66) n=69, 10mg, relative viral load reduction, mid-recovery, day 4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Wang et al., 7 Aug 2025, Double Blind Randomized Controlled Trial, China, peer-reviewed, median age 30.0, 18 authors, study period September 2023 - February 2024, trial ChiCTR2300075467.
The efficacy and safety of inhaled peptide YKYY017 for COVID-19 patients with mild illness: a phase 2 randomized controlled trial
doi:10.1038/s41467-025-62214-x
YKYY017 is a SARS-CoV-2 membrane fusion inhibitor. We report efficacy and safety of inhaled YKYY017 for COVID-19 patients with mild to moderate illness from a phase 2 trial (ChiCTR2300075467). 239 patients aged 18-75 years with mostly mild COVID-19 were randomly allocated to receive aerosol inhalation of 10 or 20 mg YKYY017 or placebo once daily. The primary endpoint is the change in SARS-CoV-2 viral load from baseline to Day 4. The mean (±SE) differences in viral load change from baseline were -0.48 ± 0.27 log 10 copies/mL (95% CI, -1.01 to 0.06) for the 20 mg group and -0.27 ± 0.27 log 10 copies/mL (95% CI, -0.79 to 0.26) for the 10 mg group, compared to the placebo group. Viral load changes at visits other than Day 4 did not differ significantly from placebo in either the 10 or 20 mg YKYY017 groups. The time to sustained symptom recovery was shorter in the 20 mg YKYY017 group (median 117.53, 95%CI 95.33 to 141.45 hours) than in the placebo group (median 143.00, 95%CI 139.17 to 186.87 hours; HR 1.552, 95%CI 1.089 to 2.214, p = 0.0151), whereas the 10 mg YKYY017 group showed a similar but not statistically significant trend compared to placebo (p = 0.0833). The time to sustained symptom alleviation was shorter in both the 20 and 10 mg YKYY017 groups than in the placebo group. The adverse events were mostly mild to moderate. The primary outcome was not met. Following a supplementary phase 1b trial, we are planning another phase 2/3 trial using a twice-daily 20 mg YKYY017 regimen to further assess efficacy and safety. Despite World Health Organization (WHO)'s declaration of ending the COVID-19 public health emergency in May 2023 1 , SARS-CoV-2 continues to cause several waves of infections annually across various geographic regions. The summer of 2024 witnessed a notable spike in COVID-19 cases, underscoring the persistent threat of COVID-19 and the need for effective treatment strategies 2 . While several small-molecule oral antivirals have gained regulatory approval, concerns about systemic adverse effects have driven the development of alternative drug delivery methods 3 . Moreover, some oral antivirals require co-administration with ritonavir, presenting clinical management challenges for patients with underlying health conditions 4 . Inhaled antiviral provides the potential for rapid and
Methods
Trial design This trial was a phase 2 randomized double-blind clinical trial to evaluate the efficacy and safety of YKYY017 aerosol inhalation in the treatment of patients with mild to moderate COVID-19 (Trial registration: ChiCTR2300075467). We enrolled patients aged 18 to 75 years with mild to moderate COVID-19. We conducted the trial in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. This trial was approved by the ethics committee in China-Japan Friendship Hospital (approval number: YM2023-043-02) and the ethics committee at each participating center. Written informed consent was obtained from all participants or their legal representatives.
Reporting summary Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Author contributions
Additional information Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s41467-025-62214-x . Correspondence and requests for materials should be addressed to Gengshen Song, Yuxian He, Chen Wang or Bin Cao. Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available. Reprints and permissions information is available at http://www.nature.com/reprints Publisher's note Springer Nature remains neutral with regard to..
References
Andrews, Herman, Gandhi, Treatments for COVID-19, Annu Rev. Med
Cevik, SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta-analysis, Lancet Microbe
Davido, Megarbane, Loubet, COVID-19 surge during summer 2024: the phantom menace?, Clin Microbiol Infect, doi:10.1016/j.cmi.2024.07.009
Jagannathan, Safety and efficacy of inhaled interferonbeta1a (SNG001) in adults with mild-to-moderate COVID-19: a randomized, controlled, phase II trial, EClinicalMedicine
Maranda, Safety and efficacy of inhaled IBIO123 for mild-tomoderate COVID-19: a randomised, double-blind, dose-ascending, placebo-controlled, phase 1/2 trial, Lancet Infect. Dis
Neant, Modeling SARS-CoV-2 viral kinetics and association with mortality in hospitalized patients from the French COVID cohort, Proc. Natl. Acad. Sci. USA
Saha, Quinones-Mateu, Das, Inhaled therapy for COVID-19: Considerations of drugs, formulations and devices, Int J. Pharm
Sahin, Antivirals and the potential benefits of orally inhaled drug administration in COVID-19 treatment, J. Pharm. Sci
Tang, Bidon, Jaimes, Whittaker, Daniel, Coronavirus membrane fusion mechanism offers a potential target for antiviral development, Antivir. Res
Wang, Shang, Wu, Wang, Ding, e-mail: songgengshen@youcareyk
Xia, Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion, Cell Res
Yang, Structural conservation among variants of the SARS-CoV-2 spike postfusion bundle, Proc. Natl. Acad. Sci. USA
Yotsuyanagi, Efficacy and safety of 5-Day Oral Ensitrelvir for patients with mild to moderate COVID-19: The SCORPIO-SR randomized clinical trial, JAMA Netw. Open
Yu, Structure-based design and characterization of novel fusion-inhibitory lipopeptides against SARS-CoV-2 and emerging variants, Emerg. Microbes Infect
Zhan, Leritrelvir for the treatment of mild or moderate COVID-19 without co-administered ritonavir: a multicentre randomised, double-blind, placebo-controlled phase 3 trial, EClini-calMedicine
Zheng, Small-molecule antiviral treatments for COVID-19: A systematic review and network meta-analysis, Int J. Antimicrob. Agents
Zhu, Comprehensive preclinical characterization of IPB29, a pan-coronavirus fusion inhibitor under clinical trials, Antivir. Res
Zhu, Development of potent pan-coronavirus fusion inhibitors with a new design strategy, MedComm
Zhu, SARS-CoV-2 fusion-inhibitory lipopeptides maintain high potency against divergent variants of concern including Omicron, Emerg. Microbes Infect
Zhu, SARS-CoV-2-derived fusion inhibitor lipopeptides exhibit highly potent and broad-spectrum activity against divergent human coronaviruses, Signal Transduct. Target Ther
Zhu, Yu, Yan, Chong, He, Design of potent membrane fusion inhibitors against SARS-CoV-2, an emerging Coronavirus with high fusogenic activity, J. Virol