Effectiveness of Early Favipiravir Therapy in Hospitalised COVID-19 Patients
et al., Advances in Virology, doi:10.1155/2022/9240941, Jun 2022
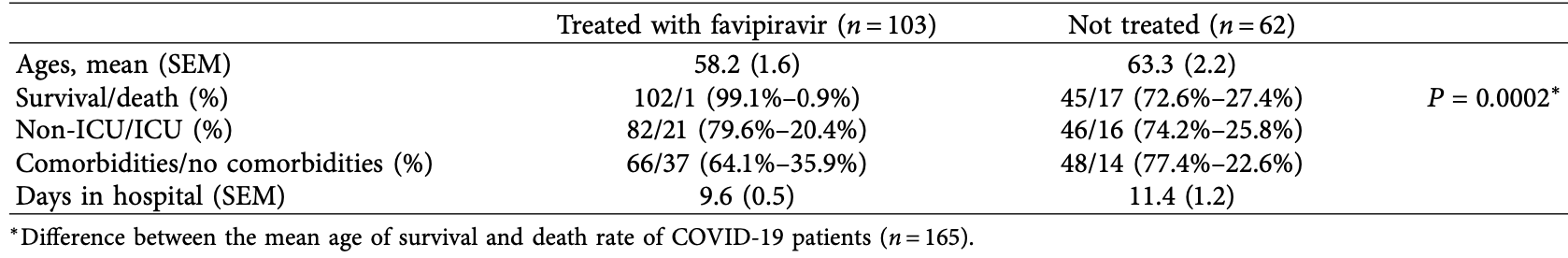
Retrospective 103 hospitalized patients in Saudi Arabia, showing lower mortality with favipiravir in unadjusted results, and greater efficacy for treatment within 3 days of admission.
Potential risks of favipiravir include kidney injury1-3, liver injury2-5, cardiovascular events5,6, pulmonary toxicity6,7, and mutagenicity, carcinogenicity, teratogenicity, embryotoxicity, and the creation of dangerous variants8-14.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with minimal group details.
|
risk of death, 96.5% lower, RR 0.04, p < 0.001, treatment 1 of 103 (1.0%), control 17 of 62 (27.4%), NNT 3.8.
|
|
risk of ICU admission, 21.0% lower, RR 0.79, p = 0.45, treatment 21 of 103 (20.4%), control 16 of 62 (25.8%), NNT 18.
|
|
hospitalization time, 15.8% lower, relative time 0.84, p < 0.001, treatment mean 9.6 (±1.2) n=102, control mean 11.4 (±1.7) n=58.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Abdulaziz et al., Clinical Features and Prognosis of Acute Kidney Injury in Hospital-Admitted Patients with COVID-19 in Egypt: A Single-Center Experience, Mansoura Medical Journal, doi:10.58775/2735-3990.1433.
2.
Ülger et al., Experimental evaluation of favipiravir (T-705)-induced liver and kidney toxicity in rats, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115472.
3.
El-Fetouh et al., Experimental Studies on Some Drugs Used in Covid-19 Treatment (Favipiravir and Dexamethasone) in Albino Rats, Journal of Advanced Veterinary Research, 13:10, www.advetresearch.com/index.php/AVR/article/view/1635.
4.
Almutairi et al., Liver Injury in Favipiravir-Treated COVID-19 Patients: Retrospective Single-Center Cohort Study, Tropical Medicine and Infectious Disease, doi:10.3390/tropicalmed8020129.
5.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
6.
Ozhan et al., Evaluation of the cardiopulmonary effects of repurposed COVID-19 therapeutics in healthy rats, Scientific Reports, doi:10.1038/s41598-025-31048-4.
7.
Ülger (B) et al., Evaluation of the effects of favipiravir (T-705) on the lung tissue of healty rats: An experimental study, Food and Chemical Toxicology, doi:10.1016/j.fct.2025.115235.
8.
Zhirnov et al., Favipiravir: the hidden threat of mutagenic action, Journal of microbiology, epidemiology and immunobiology, doi:10.36233/0372-9311-114.
9.
Waters et al., Human genetic risk of treatment with antiviral nucleoside analog drugs that induce lethal mutagenesis: the special case of molnupiravir, Environmental and Molecular Mutagenesis, doi:10.1002/em.22471.
10.
Hadj Hassine et al., Lethal Mutagenesis of RNA Viruses and Approved Drugs with Antiviral Mutagenic Activity, Viruses, doi:10.3390/v14040841.
11.
Shum, C., An investigational study into the drug-associated mutational signature in SARS-CoV-2 viruses, The University of Hong Kong, PhD Thesis, hub.hku.hk/handle/10722/344396.
12.
Shiraki et al., Convenient screening of the reproductive toxicity of favipiravir and antiviral drugs in Caenorhabditis elegans, Heliyon, doi:10.1016/j.heliyon.2024.e35331.
Tawfik et al., 29 Jun 2022, retrospective, Saudi Arabia, peer-reviewed, mean age 60.1, 8 authors, study period 3 June, 2020 - 3 November, 2020.
Contact: abdrahmankamal2@gmail.com, drsami61@gmail.com.
Effectiveness of Early Favipiravir Therapy in Hospitalised COVID-19 Patients
Advances in Virology, doi:10.1155/2022/9240941
COVID-19 is a disease caused by a novel coronavirus with no speci c, standard treatment. We investigated the clinical data of COVID-19 patients admitted to King Fahad Specialist Hospital (KFSH) in Buraydah by comparing the patients who were treated early with favipiravir (within 3 days of admission) to patients who were treated after three days of admission or not treated. 165 patients were con rmed with PCR tests and admitted to KFSH for treatment. Comorbidities contributed signi cantly to increasing the length of stay in hospital at 11.4 ± 0.8 days compared to patients with no comorbidities at 8.6 ± 0.9 days (p 0.041). A total of 103 patients were treated with favipiravir, and we found that early treatment with favipiravir (within 3 days) reduced the length of stay in hospital signi cantly (8.8 ± 1.4 days) compared to patients who were treated after 3 days (13.3 ± 4.6 days) (p 0.0015). Moreover, patients with comorbidities in both early and late treatment groups had signi cantly higher average lengths of stay in hospital (11.2 ± 0.9 days) compared to patients with no comorbidities (7.9 ± 0.7 days) (p 0.017). Interestingly, patients treated early with favipiravir (with comorbidities and without) stayed fewer days in hospital compared to those with late treatment (p 0.021; a di erence of 4.5 ± 1.9 days; and p 0.018; a di erence of 4.2 ± 1.7 days, respectively). In conclusion, our analysis indicates that early treatment with favipiravir can reduce the length of stay in hospital and improve clinical manifestations of COVID-19 patients.
Disclosure Abdulrahman Tawfik and Abdulrahman Alzahrani are cofirst authors.
Conflicts of Interest e authors declare no conflicts of interest.
References
Biswas, Rahaman, Biswas, Haque, Ibrahim, Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis, Intervirology
Bonanad, García-Blas, Tarazona-Santabalbina, e effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects, Journal of the American Medical Directors Association
Cai, Yang, Liu, Experimental treatment with favipiravir for COVID-19: an open-label control study, Engineering Times
Chen, Zhang, Huang, Favipiravir versus arbidol for clinical recovery rate in moderate and severe adult COVID-19 patients: a prospective, multicenter, open-label, randomized controlled clinical trial, Frontiers in Pharmacology
Doi, Hibino, Hase, A prospective, randomized, open-label trial of early versus late favipiravir therapy in hospitalized patients with COVID-19, Antimicrobial Agents and Chemotherapy
Ford, Vitoria, Rangaraj, Norris, Calmy et al., Systematic review of the efficacy and safety of antiretroviral drugs against SARS, MERS or COVID-19: initial assessment, Journal of the International AIDS Society
Furuta, Takahashi, Shiraki, T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections, Antiviral Research
Han, Ma, Li, Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors, Emerging Microbes & Infections
Helmy, Fawzy, Elaswad, Sobieh, Kenney et al., e COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control, Journal of Clinical Medicine
Joshi, Parkar, Ansari, Role of favipiravir in the treatment of COVID-19, International Journal of Infectious Diseases: International Journal of Infectious Diseases
Karatas, Aksoy, Ozaslan, Association of early favipiravir use with reduced COVID-19 fatality among hospitalized patients, Infection and Chemotherapy
Magleby, Westblade, Trzebucki, Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019, Clinical Infectious Diseases
Wang, Cao, Zhang, Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Research
Wu, Li, Shi, Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19), Journal of Internal Medicine
Wu, Mcgoogan, Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China, Journal of the American Medical Association
Yanai, Favipiravir: a possible pharmaceutical treatment for COVID-19, Journal of Endocrinology and Metabolism
DOI record:
{
"DOI": "10.1155/2022/9240941",
"ISSN": [
"1687-8647",
"1687-8639"
],
"URL": "http://dx.doi.org/10.1155/2022/9240941",
"abstract": "<jats:p>COVID-19 is a disease caused by a novel coronavirus with no specific, standard treatment. We investigated the clinical data of COVID-19 patients admitted to King Fahad Specialist Hospital (KFSH) in Buraydah by comparing the patients who were treated early with favipiravir (within 3 days of admission) to patients who were treated after three days of admission or not treated. 165 patients were confirmed with PCR tests and admitted to KFSH for treatment. Comorbidities contributed significantly to increasing the length of stay in hospital at 11.4 ± 0.8 days compared to patients with no comorbidities at 8.6 ± 0.9 days (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M1\">\n <mi>p</mi>\n <mo>=</mo>\n <mn>0.041</mn>\n </math>\n </jats:inline-formula>). A total of 103 patients were treated with favipiravir, and we found that early treatment with favipiravir (within 3 days) reduced the length of stay in hospital significantly (8.8 ± 1.4 days) compared to patients who were treated after 3 days (13.3 ± 4.6 days) (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M2\">\n <mi>p</mi>\n <mo>=</mo>\n <mn>0.0015</mn>\n </math>\n </jats:inline-formula>). Moreover, patients with comorbidities in both early and late treatment groups had significantly higher average lengths of stay in hospital (11.2 ± 0.9 days) compared to patients with no comorbidities (7.9 ± 0.7 days) (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M3\">\n <mi>p</mi>\n <mo>=</mo>\n <mn>0.017</mn>\n </math>\n </jats:inline-formula>). Interestingly, patients treated early with favipiravir (with comorbidities and without) stayed fewer days in hospital compared to those with late treatment (<jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M4\">\n <mi>p</mi>\n <mo>=</mo>\n <mn>0.021</mn>\n </math>\n </jats:inline-formula>; a difference of 4.5 ± 1.9 days; and <jats:inline-formula>\n <math xmlns=\"http://www.w3.org/1998/Math/MathML\" id=\"M5\">\n <mi>p</mi>\n <mo>=</mo>\n <mn>0.018</mn>\n </math>\n </jats:inline-formula>; a difference of 4.2 ± 1.7 days, respectively). In conclusion, our analysis indicates that early treatment with favipiravir can reduce the length of stay in hospital and improve clinical manifestations of COVID-19 patients.</jats:p>",
"alternative-id": [
"9240941",
"9240941"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-6697-1331",
"affiliation": [
{
"name": "Medical Cluster of Infection Prevention and Control, Alqassim, Saudi Arabia"
}
],
"authenticated-orcid": true,
"family": "Tawfik",
"given": "Abdulrahman",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Applied Medical Sciences, Applied College, Al Baha University, Al-Baha, Saudi Arabia"
}
],
"family": "Alzahrani",
"given": "Abdulrahman",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3529-4267",
"affiliation": [
{
"name": "Department of Internal Medicine, King Fahad Specialist Hospital, Alqassim, Saudi Arabia"
}
],
"authenticated-orcid": true,
"family": "Alharbi",
"given": "Sami",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Clinical Service Line, King Fahad Specialist Hospital, Alqassim, Saudi Arabia"
}
],
"family": "Almitairi",
"given": "Jamal",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "College of Medicine, University of Hail, Saudi Arabia"
}
],
"family": "Alzahrani",
"given": "Arwa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Faculty of Applied Medical Sciences, Al Baha University, Al-Baha, Saudi Arabia"
}
],
"family": "Alshehri",
"given": "Mohammed Ali",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Research Center, King Fahad Medical City, P.O. Box. 59046, Riyadh 11525, Saudi Arabia"
}
],
"family": "Aldughaim",
"given": "Mohammed S.",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-0132-558X",
"affiliation": [
{
"name": "Faculty of Applied Medical Sciences, Al Baha University, Al-Baha, Saudi Arabia"
}
],
"authenticated-orcid": true,
"family": "Alothaid",
"given": "Hani",
"sequence": "additional"
}
],
"container-title": "Advances in Virology",
"container-title-short": "Advances in Virology",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
6,
30
]
],
"date-time": "2022-06-30T06:20:09Z",
"timestamp": 1656570009000
},
"deposited": {
"date-parts": [
[
2022,
6,
30
]
],
"date-time": "2022-06-30T06:20:12Z",
"timestamp": 1656570012000
},
"editor": [
{
"affiliation": [],
"family": "Al-Shammari",
"given": "Ahmed Majeed",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2022,
6,
30
]
],
"date-time": "2022-06-30T06:42:54Z",
"timestamp": 1656571374658
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2022,
6,
29
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
6,
29
]
],
"date-time": "2022-06-29T00:00:00Z",
"timestamp": 1656460800000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/av/2022/9240941.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/av/2022/9240941.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/av/2022/9240941.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "98",
"original-title": [],
"page": "1-7",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2022,
6,
29
]
]
},
"published-print": {
"date-parts": [
[
2022,
6,
29
]
]
},
"publisher": "Hindawi Limited",
"reference": [
{
"DOI": "10.3390/jcm9041225",
"doi-asserted-by": "publisher",
"key": "1"
},
{
"DOI": "10.1002/jia2.25489",
"doi-asserted-by": "publisher",
"key": "2"
},
{
"DOI": "10.1016/j.antiviral.2009.02.198",
"doi-asserted-by": "publisher",
"key": "3"
},
{
"DOI": "10.1016/j.ijid.2020.10.069",
"doi-asserted-by": "publisher",
"key": "4"
},
{
"DOI": "10.3947/ic.2020.0149",
"doi-asserted-by": "publisher",
"key": "5"
},
{
"DOI": "10.1016/j.eng.2020.03.007",
"doi-asserted-by": "publisher",
"key": "6"
},
{
"article-title": "Saudi MoH protocol for patients suspected of/confirmed with COVID-19",
"author": "Ministry of Health",
"key": "7",
"year": "2020"
},
{
"DOI": "10.1128/AAC.01897-20",
"doi-asserted-by": "publisher",
"key": "8"
},
{
"DOI": "10.14740/jem645",
"doi-asserted-by": "publisher",
"key": "9"
},
{
"DOI": "10.1159/000512592",
"doi-asserted-by": "publisher",
"key": "10"
},
{
"DOI": "10.1016/j.jamda.2020.05.045",
"doi-asserted-by": "publisher",
"key": "11"
},
{
"DOI": "10.1111/joim.13063",
"doi-asserted-by": "publisher",
"key": "12"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"doi-asserted-by": "publisher",
"key": "13"
},
{
"DOI": "10.3389/fphar.2021.683296",
"doi-asserted-by": "publisher",
"key": "14"
},
{
"article-title": "Impact of SARS-CoV-2 viral load on risk of intubation and mortality among hospitalized patients with coronavirus disease 2019",
"author": "R. Magleby",
"journal-title": "Clinical Infectious Diseases",
"key": "15",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1770129",
"doi-asserted-by": "publisher",
"key": "16"
},
{
"DOI": "10.1001/jama.2020.2648",
"doi-asserted-by": "publisher",
"key": "17"
}
],
"reference-count": 17,
"references-count": 17,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/av/2022/9240941/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Virology"
],
"subtitle": [],
"title": "Effectiveness of Early Favipiravir Therapy in Hospitalised COVID-19 Patients",
"type": "journal-article",
"volume": "2022"
}