Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial
et al., The Lancet Respiratory Medicine, doi:10.1016/S2213-2600(21)00177-6, CYCOV, NCT04324528, Jul 2021
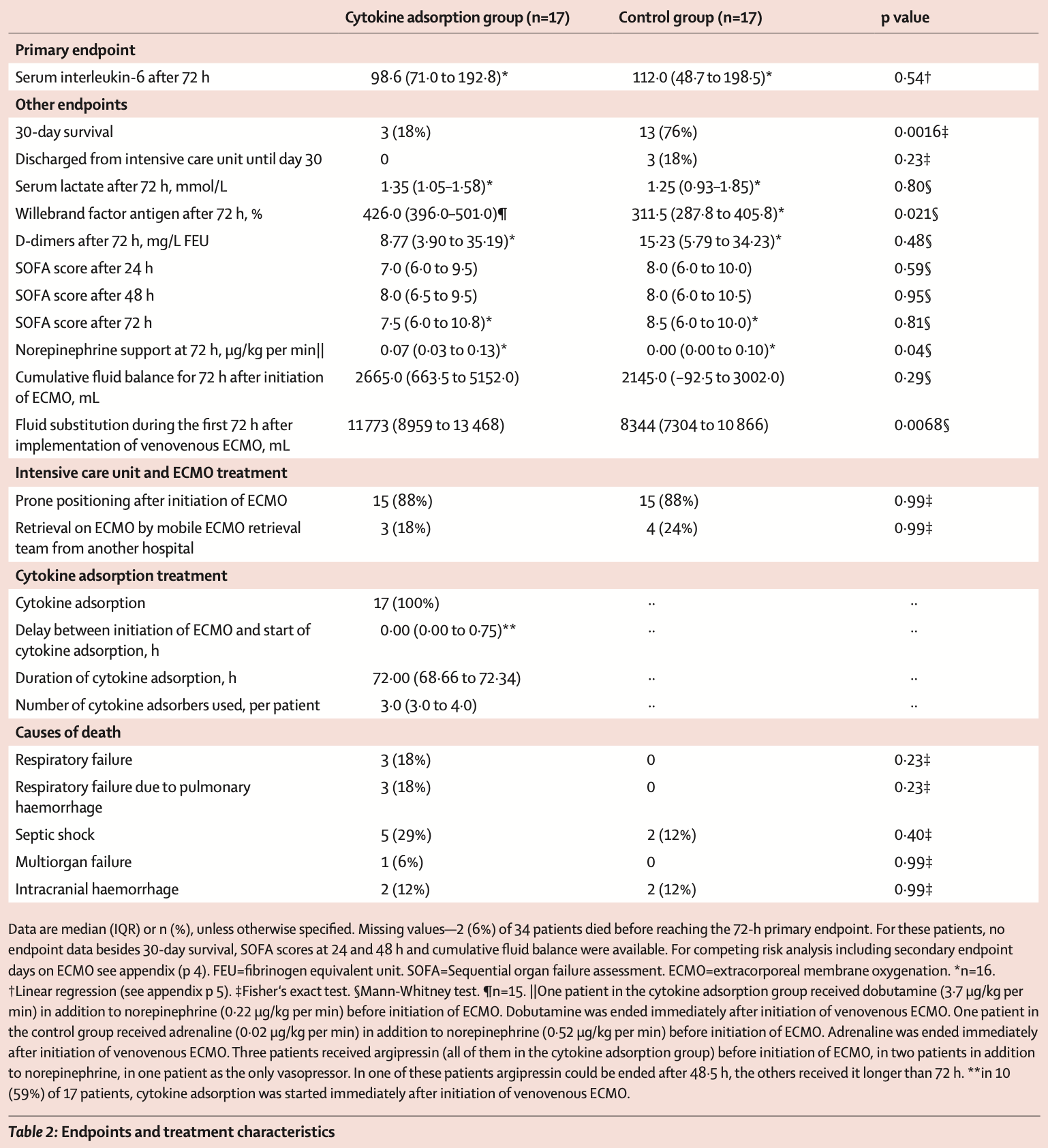
RCT 34 severe COVID-19 patients requiring extracorporeal membrane oxygenation (ECMO) showing significantly higher mortality with cytokine adsorption therapy.
|
risk of death, 250.0% higher, RR 3.50, p = 0.002, treatment 14 of 17 (82.4%), control 4 of 17 (23.5%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Supady et al., 31 Jul 2021, Randomized Controlled Trial, Germany, peer-reviewed, median age 61.0, 23 authors, study period 29 March, 2020 - 29 December, 2020, trial NCT04324528 (history) (CYCOV).
Contact: alexander.supady@uniklinikfreiburg.de.
Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial
The Lancet Respiratory Medicine, doi:10.1016/s2213-2600(21)00177-6
Background We sought to clarify the benefit of cytokine adsorption in patients with COVID-19 supported with venovenous extracorporeal membrane oxygenation (ECMO).
Methods We did a single-centre, open-label, randomised, controlled trial to investigate cytokine adsorption in adult patients with severe COVID-19 pneumonia requiring ECMO. Patients with COVID-19 selected for ECMO at the Freiburg University Medical Center (Freiburg, Germany) were randomly assigned (1:1) to receive cytokine adsorption using the CytoSorb device or not. Randomisation was computer-generated, allocation was concealed by opaque, sequentially numbered sealed envelopes. The CytoSorb device was incorporated into the ECMO circuit before connection to the patient circuit, replaced every 24 h, and removed after 72 h. The primary endpoint was serum interleukin-6 (IL-6) concentration 72 h after initiation of ECMO analysed by intention to treat. Secondary endpoints included 30-day survival. The trial is registered with ClinicalTrials.gov (NCT04324528) and the German Clinical Trials Register (DRKS00021300) and is closed. Findings From March 29, 2020, to Dec 29, 2020, of 34 patients assessed for eligibility, 17 (50%) were treated with cytokine adsorption and 17 (50%) without. Median IL-6 decreased from 357•0 pg/mL to 98•6 pg/mL in patients randomly assigned to cytokine adsorption and from 289•0 pg/mL to 112•0 pg/mL in the control group after 72 h. One patient in each group died before 72 h. Adjusted mean log IL-6 concentrations after 72 h were 0•30 higher in the cytokine adsorption group (95% CI -0•70 to 1•30, p=0•54). Survival after 30 days was three (18%) of 17 with cytokine adsorption and 13 (76%) of 17 without cytokine adsorption (p=0•0016). Interpretation Early initiation of cytokine adsorption in patients with severe COVID-19 and venovenous ECMO did not reduce serum IL-6 and had a negative effect on survival. Cytokine adsorption should not be used during the first days of ECMO support in COVID-19.
Contributors ASu was principal investigator of the trial, he wrote the study protocol, designed and supervised the trial, and wrote the first draft of the manuscript. ASu and DD accessed and verified the data. Statistical analyses were done by EW, EG, and AS. MR, AL, TN, TZ, FF, SMü, MK, CBe, SMa, GT, AF, KK, ASe, PS, VZ, CBo, PMB, DS, TW, and DD supported acquisition, analysis, and interpretation of the data. All authors had full access to all the data and the corresponding author had final responsibility for the decision to submit for publication. All authors read and approved the final manuscript.
Declarations of interests All authors have completed the ICMJE form (available on request from the corresponding author). ASu reports research grants and lecture fees from CytoSorbents and lecture fees from Abiomed, both outside of the submitted work. MR reports funding by the IMM-PACT-Programme for Clinician Scientists, Department of Medicine II, Medical Center, University of Freiburg and Faculty of Medicine, funded by the Deutsche Forschungsgemeinschaft (German Research Foundation)-413517907 and financial support from CytoSorbents for attending a scientific meeting. AL reports a research grant from the German Center for infectious diseases, outside of the submitted work and is a member of SFB1425, funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation #422681845). SMa reports honoraria from CytoSorbents for a presentation during a scientific workshop...
References
Akil, Ziegeler, Reichelt, Combined Use of CytoSorb and ECMO in patients with severe pneumogenic sepsis, Thorac Cardiovasc Surg
Akin, Garcheva, Sieweke, Early use of hemoadsorption in patients after out-of hospital cardiac arrest -a matched pair analysis, PLoS One
Barbaro, Maclaren, Boonstra, Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry, Lancet
Brouwer, Duran, Kuijper, Ince, Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day allcause mortality in ICU patients with septic shock: a propensityscore-weighted retrospective study, Crit Care
Calabrò, Febres, Recca, Blood purification with CytoSorb in critically ill patients: single-center preliminary experience, Artif Organs
Cummings, Baldwin, Abrams, Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study, Lancet
Fajgenbaum, June, Cytokine storm, N Engl J Med
Friesecke, Träger, Schittek, International registry on the use of the CytoSorb® adsorber in ICU patients: study protocol and preliminary results, Med Klin Intensivmed Notfmed
Greil, Roether, Rosée, Grimbacher, Duerschmied et al., Rescue of cytokine storm due to HLH by hemoadsorption in a CTLA4-deficient patient, J Clin Immunol
Henry, COVID-19, ECMO, and lymphopenia: a word of caution, Lancet Respir Med
Karagiannidis, Mostert, Hentschker, Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study, Lancet Respir Med
Kogelmann, Jarczak, Scheller, Drüner, Hemoadsorption by CytoSorb in septic patients: a case series, Crit Care
Kogelmann, Scheller, Drüner, Jarczak, Use of hemoadsorption in sepsis-associated ECMO-dependent severe ARDS: A case series, J Intensive Care Soc
Kox, Waalders, Kooistra, Gerretsen, Pickkers, Cytokine levels in critically Ill patients with COVID-19 and other conditions, JAMA
Lebreton, Dorgham, Quentric, Combes, Gorochov et al., Longitudinal cytokine profiling in severe COVID-19 patients on ECMO and haemoadsorption, Am J Respir Crit Care Med
Leisman, Ronner, Pinotti, Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes, Lancet Respir Med
Mcelvaney, Mcevoy, Mcelvaney, Characterization of the Inflammatory Response to Severe COVID-19 Illness, Am J Respir Crit Care Med
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Onder, Rezza, Brusaferro, Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy, JAMA
Poli, Alberio, Bauer-Doerries, Cytokine clearance with CytoSorb® during cardiac surgery: a pilot randomized controlled trial, Crit Care
Poli, Rimmelé, Schneider, Hemoadsorption with CytoSorb ®, Intensive Care Med
Rieder, Duerschmied, Zahn, Cytokine adsorption in severe acute respiratory failure requiring veno-venous extracorporeal membrane oxygenation, ASAIO J
Rieder, Goller, Jeserich, Rate of venous thromboembolism in a prospective all-comers cohort with COVID-19, J Thromb Thrombolysis
Rieder, Schubach, Schmoor, Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation: protocol for a randomised, controlled, open-label intervention, multicentre trial, BMJ Open
Rieder, Wengenmayer, Staudacher, Duerschmied, Supady, Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation, Crit Care
Rieder, Wirth, Pollmeier, Serum protein profiling reveals a specific upregulation of the immunomodulatory protein progranulin in COVID-19, J Infect Dis
Rieder, Zahn, Benk, Cytokine adsorption in a patient with severe coronavirus disease 2019 related acute respiratory distress syndrome requiring extracorporeal membrane oxygenation therapy: a case report, Artif Organs
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med
Schmidt, Hajage, Lebreton, Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study, Lancet Respir Med
Schädler, Pausch, Heise, The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial, PLoS One
Sinha, Matthay, Calfee, Is a "Cytokine storm" relevant to COVID-19?, JAMA Intern Med
Vincent, Moreno, Takala, The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/ failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine, Intensive Care Med
Wichmann, Sperhake, Lütgehetmann, Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study, Ann Intern Med
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
Zuccari, Damiani, Domizi, Changes in cytokines, haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with CytoSorb, Blood Purif
DOI record:
{
"DOI": "10.1016/s2213-2600(21)00177-6",
"ISSN": [
"2213-2600"
],
"URL": "http://dx.doi.org/10.1016/S2213-2600(21)00177-6",
"alternative-id": [
"S2213260021001776"
],
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "The Lancet Respiratory Medicine"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/S2213-2600(21)00177-6"
},
{
"label": "CrossRef DOI link to the associated document",
"name": "associatedlink",
"value": "https://doi.org/10.1016/S2213-2600(21)00207-1"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2021 Elsevier Ltd. All rights reserved."
}
],
"author": [
{
"affiliation": [],
"family": "Supady",
"given": "Alexander",
"sequence": "first"
},
{
"affiliation": [],
"family": "Weber",
"given": "Enya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rieder",
"given": "Marina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lother",
"given": "Achim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Niklaus",
"given": "Tim",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zahn",
"given": "Timm",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frech",
"given": "Franziska",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Müller",
"given": "Sissi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kuhl",
"given": "Moritz",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benk",
"given": "Christoph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maier",
"given": "Sven",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Trummer",
"given": "Georg",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Flügler",
"given": "Annabelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Krüger",
"given": "Kirsten",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sekandarzad",
"given": "Asieb",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Stachon",
"given": "Peter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zotzmann",
"given": "Viviane",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bode",
"given": "Christoph",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Biever",
"given": "Paul M",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Staudacher",
"given": "Dawid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wengenmayer",
"given": "Tobias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Graf",
"given": "Erika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Duerschmied",
"given": "Daniel",
"sequence": "additional"
}
],
"container-title": "The Lancet Respiratory Medicine",
"container-title-short": "The Lancet Respiratory Medicine",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"clinicalkey.fr",
"clinicalkey.jp",
"clinicalkey.com.au",
"clinicalkey.es",
"clinicalkey.com",
"thelancet.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2021,
5,
14
]
],
"date-time": "2021-05-14T22:36:26Z",
"timestamp": 1621031786000
},
"deposited": {
"date-parts": [
[
2023,
5,
2
]
],
"date-time": "2023-05-02T06:43:28Z",
"timestamp": 1683009808000
},
"indexed": {
"date-parts": [
[
2025,
5,
6
]
],
"date-time": "2025-05-06T10:11:12Z",
"timestamp": 1746526272491
},
"is-referenced-by-count": 154,
"issue": "7",
"issued": {
"date-parts": [
[
2021,
7
]
]
},
"journal-issue": {
"issue": "7",
"published-print": {
"date-parts": [
[
2021,
7
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-017",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-037",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-012",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-029",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
},
{
"URL": "https://doi.org/10.15223/policy-004",
"content-version": "stm-asf",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021001776?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2213260021001776?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "755-762",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2021,
7
]
]
},
"published-print": {
"date-parts": [
[
2021,
7
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"DOI": "10.1016/S0140-6736(20)31189-2",
"article-title": "Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study",
"author": "Cummings",
"doi-asserted-by": "crossref",
"first-page": "1763",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(21)00177-6_bib1",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30316-7",
"article-title": "Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study",
"author": "Karagiannidis",
"doi-asserted-by": "crossref",
"first-page": "853",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00177-6_bib2",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)32008-0",
"article-title": "Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry",
"author": "Barbaro",
"doi-asserted-by": "crossref",
"first-page": "1071",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(21)00177-6_bib3",
"volume": "396",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30328-3",
"article-title": "Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome associated with COVID-19: a retrospective cohort study",
"author": "Schmidt",
"doi-asserted-by": "crossref",
"first-page": "1121",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00177-6_bib4",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30119-3",
"article-title": "COVID-19, ECMO, and lymphopenia: a word of caution",
"author": "Henry",
"doi-asserted-by": "crossref",
"first-page": "e24",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00177-6_bib5",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202005-1583OC",
"article-title": "Characterization of the Inflammatory Response to Severe COVID-19 Illness",
"author": "McElvaney",
"doi-asserted-by": "crossref",
"first-page": "812",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/S2213-2600(21)00177-6_bib6",
"volume": "202",
"year": "2020"
},
{
"DOI": "10.1056/NEJMra2026131",
"article-title": "Cytokine storm",
"author": "Fajgenbaum",
"doi-asserted-by": "crossref",
"first-page": "2255",
"journal-title": "N Engl J Med",
"key": "10.1016/S2213-2600(21)00177-6_bib7",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"article-title": "COVID-19: consider cytokine storm syndromes and immunosuppression",
"author": "Mehta",
"doi-asserted-by": "crossref",
"first-page": "1033",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(21)00177-6_bib8",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"article-title": "Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China",
"author": "Ruan",
"doi-asserted-by": "crossref",
"first-page": "846",
"journal-title": "Intensive Care Med",
"key": "10.1016/S2213-2600(21)00177-6_bib9",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"article-title": "Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study",
"author": "Zhou",
"doi-asserted-by": "crossref",
"first-page": "1054",
"journal-title": "Lancet",
"key": "10.1016/S2213-2600(21)00177-6_bib10",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1186/s13054-019-2588-1",
"article-title": "Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study",
"author": "Brouwer",
"doi-asserted-by": "crossref",
"first-page": "317",
"journal-title": "Crit Care",
"key": "10.1016/S2213-2600(21)00177-6_bib11",
"volume": "23",
"year": "2019"
},
{
"DOI": "10.1007/s00063-017-0342-5",
"article-title": "International registry on the use of the CytoSorb® adsorber in ICU patients: study protocol and preliminary results",
"author": "Friesecke",
"doi-asserted-by": "crossref",
"first-page": "699",
"journal-title": "Med Klin Intensivmed Notfmed",
"key": "10.1016/S2213-2600(21)00177-6_bib12",
"volume": "114",
"year": "2019"
},
{
"DOI": "10.1007/s10875-017-0377-7",
"article-title": "Rescue of cytokine storm due to HLH by hemoadsorption in a CTLA4-deficient patient",
"author": "Greil",
"doi-asserted-by": "crossref",
"first-page": "273",
"journal-title": "J Clin Immunol",
"key": "10.1016/S2213-2600(21)00177-6_bib13",
"volume": "37",
"year": "2017"
},
{
"DOI": "10.1007/s00134-018-5464-6",
"article-title": "Hemoadsorption with CytoSorb®",
"author": "Poli",
"doi-asserted-by": "crossref",
"first-page": "236",
"journal-title": "Intensive Care Med",
"key": "10.1016/S2213-2600(21)00177-6_bib14",
"volume": "45",
"year": "2019"
},
{
"DOI": "10.1111/aor.13327",
"article-title": "Blood purification with CytoSorb in critically ill patients: single-center preliminary experience",
"author": "Calabrò",
"doi-asserted-by": "crossref",
"first-page": "189",
"journal-title": "Artif Organs",
"key": "10.1016/S2213-2600(21)00177-6_bib15",
"volume": "43",
"year": "2019"
},
{
"DOI": "10.1097/MAT.0000000000001302",
"article-title": "Cytokine adsorption in severe acute respiratory failure requiring veno-venous extracorporeal membrane oxygenation",
"author": "Rieder",
"doi-asserted-by": "crossref",
"first-page": "332",
"journal-title": "ASAIO J",
"key": "10.1016/S2213-2600(21)00177-6_bib16",
"volume": "67",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03130-y",
"article-title": "Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation",
"author": "Rieder",
"doi-asserted-by": "crossref",
"first-page": "435",
"journal-title": "Crit Care",
"key": "10.1016/S2213-2600(21)00177-6_bib18",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1111/aor.13805",
"article-title": "Cytokine adsorption in a patient with severe coronavirus disease 2019 related acute respiratory distress syndrome requiring extracorporeal membrane oxygenation therapy: a case report",
"author": "Rieder",
"doi-asserted-by": "crossref",
"first-page": "191",
"journal-title": "Artif Organs",
"key": "10.1016/S2213-2600(21)00177-6_bib19",
"volume": "45",
"year": "2020"
},
{
"article-title": "Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy",
"author": "Onder",
"first-page": "1775",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00177-6_bib20",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1007/BF01709751",
"article-title": "The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine",
"author": "Vincent",
"doi-asserted-by": "crossref",
"first-page": "707",
"journal-title": "Intensive Care Med",
"key": "10.1016/S2213-2600(21)00177-6_bib21",
"volume": "22",
"year": "1996"
},
{
"DOI": "10.1186/s13054-019-2399-4",
"article-title": "Cytokine clearance with CytoSorb® during cardiac surgery: a pilot randomized controlled trial",
"author": "Poli",
"doi-asserted-by": "crossref",
"first-page": "108",
"journal-title": "Crit Care",
"key": "10.1016/S2213-2600(21)00177-6_bib22",
"volume": "23",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0187015",
"article-title": "The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: a randomized controlled trial",
"author": "Schädler",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(21)00177-6_bib23",
"volume": "12",
"year": "2017"
},
{
"DOI": "10.1159/000502540",
"article-title": "Changes in cytokines, haemodynamics and microcirculation in patients with sepsis/septic shock undergoing continuous renal replacement therapy and blood purification with CytoSorb",
"author": "Zuccari",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "Blood Purif",
"key": "10.1016/S2213-2600(21)00177-6_bib24",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1164/rccm.202011-4140LE",
"article-title": "Longitudinal cytokine profiling in severe COVID-19 patients on ECMO and haemoadsorption",
"author": "Lebreton",
"doi-asserted-by": "crossref",
"journal-title": "Am J Respir Crit Care Med",
"key": "10.1016/S2213-2600(21)00177-6_bib25",
"year": "2021"
},
{
"DOI": "10.1177/1751143718818992",
"article-title": "Use of hemoadsorption in sepsis-associated ECMO-dependent severe ARDS: A case series",
"author": "Kogelmann",
"doi-asserted-by": "crossref",
"first-page": "183",
"journal-title": "J Intensive Care Soc",
"key": "10.1016/S2213-2600(21)00177-6_bib26",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1055/s-0040-1708479",
"article-title": "Combined Use of CytoSorb and ECMO in patients with severe pneumogenic sepsis",
"author": "Akil",
"doi-asserted-by": "crossref",
"first-page": "246",
"journal-title": "Thorac Cardiovasc Surg",
"key": "10.1016/S2213-2600(21)00177-6_bib27",
"volume": "69",
"year": "2021"
},
{
"DOI": "10.1186/s13054-017-1662-9",
"article-title": "Hemoadsorption by CytoSorb in septic patients: a case series",
"author": "Kogelmann",
"doi-asserted-by": "crossref",
"first-page": "74",
"journal-title": "Crit Care",
"key": "10.1016/S2213-2600(21)00177-6_bib28",
"volume": "21",
"year": "2017"
},
{
"DOI": "10.1371/journal.pone.0241709",
"article-title": "Early use of hemoadsorption in patients after out-of hospital cardiac arrest - a matched pair analysis",
"author": "Akin",
"doi-asserted-by": "crossref",
"journal-title": "PLoS One",
"key": "10.1016/S2213-2600(21)00177-6_bib29",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.7326/M20-2003",
"article-title": "Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study",
"author": "Wichmann",
"doi-asserted-by": "crossref",
"first-page": "268",
"journal-title": "Ann Intern Med",
"key": "10.1016/S2213-2600(21)00177-6_bib30",
"volume": "173",
"year": "2020"
},
{
"DOI": "10.1007/s11239-020-02202-8",
"article-title": "Rate of venous thromboembolism in a prospective all-comers cohort with COVID-19",
"author": "Rieder",
"doi-asserted-by": "crossref",
"first-page": "558",
"journal-title": "J Thromb Thrombolysis",
"key": "10.1016/S2213-2600(21)00177-6_bib31",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.17052",
"article-title": "Cytokine levels in critically Ill patients with COVID-19 and other conditions",
"author": "Kox",
"doi-asserted-by": "crossref",
"journal-title": "JAMA",
"key": "10.1016/S2213-2600(21)00177-6_bib32",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(20)30404-5",
"article-title": "Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes",
"author": "Leisman",
"doi-asserted-by": "crossref",
"first-page": "1233",
"journal-title": "Lancet Respir Med",
"key": "10.1016/S2213-2600(21)00177-6_bib33",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1001/jamainternmed.2020.3313",
"article-title": "Is a “Cytokine storm” relevant to COVID-19?",
"author": "Sinha",
"doi-asserted-by": "crossref",
"first-page": "1152",
"journal-title": "JAMA Intern Med",
"key": "10.1016/S2213-2600(21)00177-6_bib34",
"volume": "180",
"year": "2020"
},
{
"DOI": "10.1093/infdis/jiaa741",
"article-title": "Serum protein profiling reveals a specific upregulation of the immunomodulatory protein progranulin in COVID-19",
"author": "Rieder",
"doi-asserted-by": "crossref",
"first-page": "775",
"journal-title": "J Infect Dis",
"key": "10.1016/S2213-2600(21)00177-6_bib35",
"volume": "223",
"year": "2021"
},
{
"DOI": "10.1136/bmjopen-2020-043345",
"article-title": "Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation: protocol for a randomised, controlled, open-label intervention, multicentre trial",
"author": "Rieder",
"doi-asserted-by": "crossref",
"journal-title": "BMJ Open",
"key": "10.1016/S2213-2600(21)00177-6_bib36",
"volume": "11",
"year": "2021"
}
],
"reference-count": 35,
"references-count": 35,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2213260021001776"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1016/elsevier_cm_policy",
"updated-by": [
{
"DOI": "10.1016/s2213-2600(21)00273-3",
"label": "Erratum",
"source": "publisher",
"type": "erratum",
"updated": {
"date-parts": [
[
2021,
7,
1
]
],
"date-time": "2021-07-01T00:00:00Z",
"timestamp": 1625097600000
}
}
],
"volume": "9"
}