Effects of chlorhexidine gluconate and povidone-iodine mouthwash on cycle threshold values in patients infected with SARS-CoV-2
et al., Dental and Medical Problems, doi:10.17219/dmp/192493, Apr 2025
53rd treatment shown to reduce risk in
November 2023, now with p < 0.00000000001 from 5 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Prospective study of 45 COVID-19 patients showing improved viral clearance with chlorhexidine gluconate and povidone-iodine mouthwash use.
Study covers chlorhexidine and povidone-iodine.
|
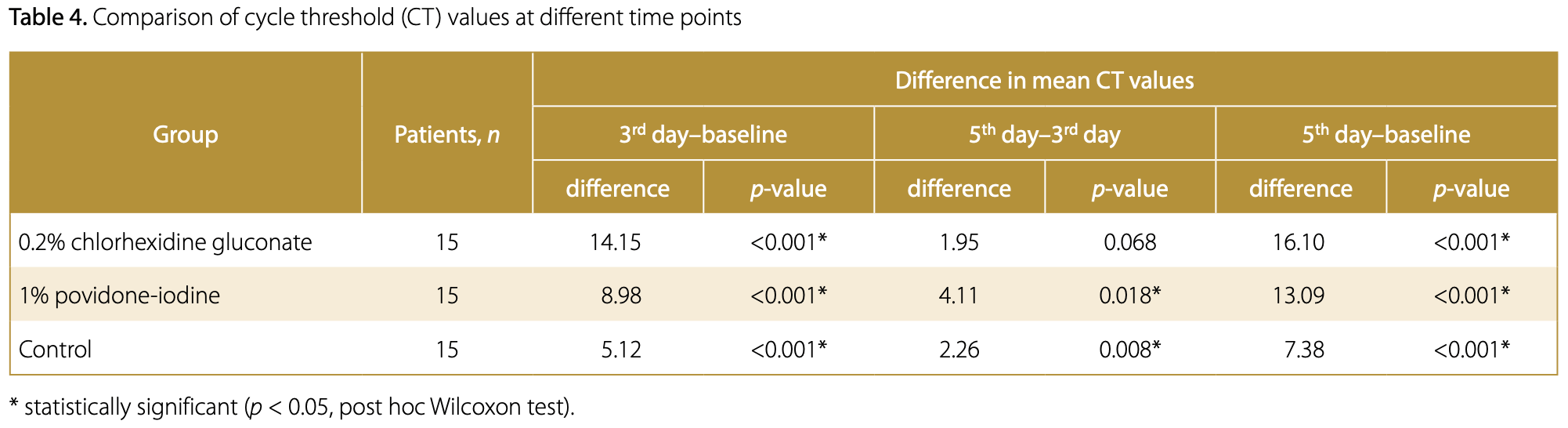
viral load, 54.2% lower, relative load 0.46, p = 0.001, treatment 15, control 15, relative increase in Ct value, day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Sulistyani et al., 30 Apr 2025, prospective, Indonesia, peer-reviewed, 8 authors.
Contact: liliesdwi_s@yahoo.co.id.
Effects of chlorhexidine gluconate and povidone-iodine mouthwash on cycle threshold values in patients infected with SARS-CoV-2
Dental and Medical Problems, doi:10.17219/dmp/192493
Background. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants exhibit different phenotypes and clinical manifestations in comparison to non-mutated viruses. Spike gene target failure (SGTF) is a characteristic feature of the gene in a novel variant that is recognized as highly transmissible. Several studies have demonstrated the virucidal effects of mouthwashes on SARS-CoV-2. Moreover, mouthwashes have proven beneficial for patients undergoing oral and maxillofacial surgery. Objectives. The present study aimed to analyze the effects of 2 different types of mouthwash (0.2% chlorhexidine gluconate and 1% povidone-iodine) on the cycle threshold (CT) values in coronavirus disease 2019 (COVID-19) patients with and without SGTF. Material and methods. This single-blind, non-randomized controlled clinical trial comprised 45 patients who were divided into 3 groups based on the intervention method: 0.2% chlorhexidine gluconate mouthwash; 1% povidone-iodine mouthwash; and mineral water (control group). The patients were instructed to gargle with the assigned solution 3 times a day for 5 days. Reverse transcription polymerase chain reaction (RT-PCR) tests were conducted at the time of initial diagnosis and on days 3 and 5. A normality test (Shapiro-Wilk test) was performed. Consequently, the non-parametric Friedman test was used. Results. The analysis revealed that the subjects who utilized mouthwashes exhibited higher CT values in comparison to the control group. Furthermore, 73% of patients who used 0.2% chlorhexidine gluconate presented with increased CT values, as indicated by a negative RT-PCR test on the 3 rd day. Conclusions. Gargling with 0.2% chlorhexidine gluconate or 1% povidone-iodine for 30 s for at least 3 days has been demonstrated to increase CT values in both SGTF and non-SGTF COVID-19 patients. Hence, using the mouthwash may be considered for preoperative use in patients undergoing oral and maxillofacial surgery.
Conflict of interest
None declared
Conclusions Gargling with 0.2% chlorhexidine gluconate or 1% povidone-iodine for 30 s for at least 3 days was shown to reduce the viral load of SARS-CoV-2. An increase in CT values was observed in patients with and without SGTF, indicating that mouthwash is effective against all SARS-CoV-2 variants. Gargling with 0.2% chlorhexidine gluconate or 1% povidone-iodine could be considered as an initial protocol prior to oral and maxillofacial surgical procedures in patients with COVID-19.
Trial registration This study was registered in the International Standard Randomised Controlled Trial Number (ISRCTN) registry under No. ISRCTN13090248.
Ethics approval and consent to participate The study protocol was reviewed and approved by the Health Ethics Committee of Persahabatan Central General Hospital, Jakarta, Indonesia (protocol No. 73/KEPK-RSUPP/08/2022).
Consent for publication Not applicable.
Use of AI and AI-assisted technologies Not applicable.
References
Boyapati, Dhulipalla, Kolaparthy, Bodduru, COVID-19 and oral implications: An updated review, J Oral Maxillofac Pathol, doi:10.4103/jomfp.jomfp_198_21
Boyapati, Peeta, Dhulipalla, Kolaparthy, Adurty et al., Comparative evaluation of the efficacy of probiotic, Aloe vera, povidine-iodine, and chlorhexidine mouthwashes in the treatment of gingival inflammation: A randomized controlled trial, Dent Med Probl, doi:10.17219/dmp/156425
Case, Bailey, Kim, Chen, Diamond, Growth, detection, quantification, and inactivation of SARS-CoV-2, Virology, doi:10.1016/j.virol.2020.05.015
Elzein, Sater, Fakhreddine, In vivo evaluation of the virucidal efficacy of chlorhexidine and povidone-iodine mouthwashes against salivary SARS-CoV-2, J Evid Based Dent Pract, doi:10.1101/2021.03.07.21252302
Herrera, Serrano, Roldán, Sanz, Is the oral cavity relevant in SARS-CoV-2 pandemic?, Clin Oral Investig, doi:10.1007/s00784-020-03413-2
Huang, Huang, Use of chlorhexidine to eradicate oropharyngeal SARS-CoV-2 in COVID-19 patients, J Med Virol, doi:10.1002/jmv.26954
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Karyono, Wicaksana, Current prevalence, characteristics, and comorbidities of patients with COVID-19 in Indonesia, J Community Empowerment Health, doi:10.22146/jcoemph.57325
Lippi, Simundic, Plebani, Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19), Clin Chem Lab Med, doi:10.1515/cclm-2020-0285
Liu, Liao, Qian, Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, Emerg Infect Dis, doi:10.3201/EID2606.200239
Megasari, Utsumi, Yamani, Seroepidemiological study of SARS-CoV-2 infection in East Java, Indonesia, PloS One, doi:10.1371/journal.pone.0251234
Meister, Brüggemann, Todt, Erratum to: Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2, J Infect Dis, doi:10.1093/infdis/jiaa539
Rao, Manissero, Steele, Pareja, A systematic review of the clinical utility of cycle threshold values in the context of COVID-19, Infect Dis Ther, doi:10.1007/s40121-020-00324-3
Robinot, Hubert, De Melo, SARS-CoV-2 infection induces the dedifferentiation of multiciliated cells and impairs mucociliary clearance, Nat Commun, doi:10.1038/s41467-021-24521-x
Satomura, Kitamura, Kawamura, Prevention of upper respiratory tract infections by gargling: A randomized trial, Am J Prev Med, doi:10.1016/j.amepre.2005.06.013
Shankar, Saha, Jamir, Kakkar, Protection at portal of entry (PPE) with povidone iodine for COVID-19, Int J Med Public Health, doi:10.5530/ijmedph.2020.4.36
Singh, Sharma, Singh, Singh, Mangal et al., Nasopharyngeal wash in preventing and treating upper respiratory tract infections: Could it prevent COVID-19?, Lung India, doi:10.4103/lungindia.lungindia_241_20
Soundarajan, Rajasekar, Antibacterial and anti-inflammatory effects of a novel herb-mediated nanocomposite mouthwash in plaque-induced gingivitis: A randomized controlled trial, Dent Med Probl, doi:10.17219/dmp/150728
Torretta, Zuccotti, Cristofaro, Diagnosis of SARS-CoV-2 by RT-PCR using different sample sources: Review of the literature, Ear Nose Throat J, doi:10.1177/0145561320953231
Tsai, Wu, Possible beneficial role of throat gargling in the coronavirus disease pandemic, Public Health, doi:10.1016/j.puhe.2020.05.055
Walker, Pritchard, House, COVID-19 Infection Survey Team. Ct threshold values, a proxy for viral load in community SARS-CoV-2 cases, demonstrate wide variation across populations and over time, ELife, doi:10.7554/eLife.64683
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China, JAMA, doi:10.1001/jama.2020.1585
Wolter, Jassat, Walaza, Early assessment of the clinical severity of the SARS-CoV-2 Omicron variant in South Africa: A data linkage study, Lancet, doi:10.1016/S0140-6736(22)00017-4
Yoon, Yoon, Song, Clinical significance of a high SARS-CoV-2 viral load in the saliva, J Korean Med Sci, doi:10.3346/jkms.2020.35.e195
DOI record:
{
"DOI": "10.17219/dmp/192493",
"ISSN": [
"1644-387X",
"2300-9020"
],
"URL": "http://dx.doi.org/10.17219/dmp/192493",
"alternative-id": [
"192493"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0001-5542-6787",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sulistyani",
"given": "Lilies Dwi",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0001-9839-6733",
"affiliation": [],
"authenticated-orcid": false,
"family": "Rizki",
"given": "Teuku Zulfahmi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0009-0001-5249-9945",
"affiliation": [],
"authenticated-orcid": false,
"family": "Haryanto",
"given": "Budi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6182-5688",
"affiliation": [],
"authenticated-orcid": false,
"family": "Julia",
"given": "Vera",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-0786-7750",
"affiliation": [],
"authenticated-orcid": false,
"family": "Badeges",
"given": "Arfan",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6407-4576",
"affiliation": [],
"authenticated-orcid": false,
"family": "Ariawan",
"given": "Dwi",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6910-3476",
"affiliation": [],
"authenticated-orcid": false,
"family": "Latief",
"given": "Mohammad Adhitya",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-8590-2983",
"affiliation": [],
"authenticated-orcid": false,
"family": "Utomo",
"given": "Yudy Ardilla",
"sequence": "additional"
}
],
"container-title": "Dental and Medical Problems",
"container-title-short": "Dent. Med. Probl.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2025,
5,
9
]
],
"date-time": "2025-05-09T08:13:18Z",
"timestamp": 1746778398000
},
"deposited": {
"date-parts": [
[
2025,
5,
9
]
],
"date-time": "2025-05-09T08:13:18Z",
"timestamp": 1746778398000
},
"indexed": {
"date-parts": [
[
2025,
5,
10
]
],
"date-time": "2025-05-10T04:02:27Z",
"timestamp": 1746849747986,
"version": "3.40.5"
},
"is-referenced-by-count": 0,
"issue": "2",
"issued": {
"date-parts": [
[
2025,
4,
30
]
]
},
"journal-issue": {
"issue": "2",
"published-print": {
"date-parts": [
[
2025
]
]
}
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/3.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
30
]
],
"date-time": "2025-04-30T00:00:00Z",
"timestamp": 1745971200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/3.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
30
]
],
"date-time": "2025-04-30T00:00:00Z",
"timestamp": 1745971200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/3.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
4,
30
]
],
"date-time": "2025-04-30T00:00:00Z",
"timestamp": 1745971200000
}
}
],
"member": "6900",
"original-title": [],
"page": "217-223",
"prefix": "10.17219",
"published": {
"date-parts": [
[
2025,
4,
30
]
]
},
"published-online": {
"date-parts": [
[
2025,
4,
30
]
]
},
"publisher": "Wroclaw Medical University",
"reference-count": 0,
"references-count": 0,
"relation": {},
"resource": {
"primary": {
"URL": "https://dmp.umw.edu.pl/en/article/2025/62/2/217/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Effects of chlorhexidine gluconate and povidone-iodine mouthwash on cycle threshold values in patients infected with SARS-CoV-2",
"type": "journal-article"
}
