Oral antiviral clevudine compared with placebo in Korean COVID-19 patients with moderate severity
et al., medRxiv, doi:10.1101/2021.12.09.21267566, BK-CLV-201, NCT04347915, Dec 2021
RCT 61 moderate COVID-19 patients in Korea showing no significant difference in viral clearance or clinical outcomes with clevudine compared to placebo.
|
risk of no recovery, 743.9% higher, RR 8.44, p = 0.16, treatment 5 of 41 (12.2%), control 0 of 20 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of no viral clearance, 26.8% lower, RR 0.73, p = 0.56, treatment 12 of 41 (29.3%), control 8 of 20 (40.0%), NNT 9.3, day 29.
|
|
risk of no viral clearance, 22.0% higher, RR 1.22, p = 0.59, treatment 20 of 41 (48.8%), control 8 of 20 (40.0%), day 22.
|
|
risk of no viral clearance, 5.7% lower, RR 0.94, p = 1.00, treatment 29 of 41 (70.7%), control 15 of 20 (75.0%), NNT 23, day 15.
|
|
risk of no viral clearance, 5.1% lower, RR 0.95, p = 1.00, treatment 35 of 41 (85.4%), control 18 of 20 (90.0%), NNT 22, day 11.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Song et al., 14 Dec 2021, Single Blind Randomized Controlled Trial, placebo-controlled, South Korea, preprint, mean age 60.0, 13 authors, trial NCT04347915 (history) (BK-CLV-201).
Contact: wjkim@korea.ac.kr.
Oral antiviral clevudine compared with placebo in Korean COVID-19 patients with moderate severity
doi:10.1101/2021.12.09.21267566
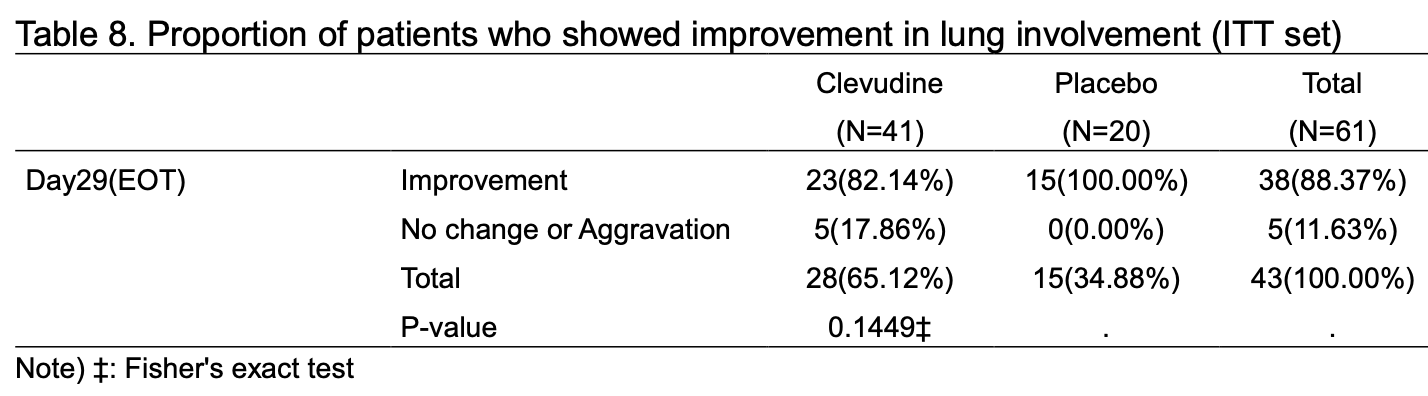
Background: Clevudine, an antiviral drug for chronic hepatitis B virus infection, is expected to inhibit the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. Therefore, we conducted a prospective, single-blind, proof of concept clinical study to examine the antiviral efficacy and safety of clevudine compared to placebo in Korean corona virus disease 19 (COVID-19) patients with moderate severity. Methods: Adults with confirmed SARS-CoV-2 infection and symptom onset within 7 days were randomized 2:1 to 120 mg clevudine or placebo to receive one of treatments orally once-daily for 14 days. Antiviral efficacy outcomes were the proportion of patients with real-time reverse transcription polymerase chain reaction (RT-PCR) negative result for SARS-CoV-2 infection and cycle threshold (Ct) value changes from baseline. Clinical efficacy outcomes included proportion of patients who showed improvement in lung involvement by imaging tests, proportion of patients with normal body temperature, proportion of patients with normal oxygen saturation, and the changes in C-reactive protein (CRP) from baseline. Safety outcomes included changes in clinical laboratory tests, vital signs measurement, and physical examination from baseline, and incidence of adverse events.
Results: The proportion of patients with real-time RT-PCR negative test and Ct value changes showed no significant difference between clevudine group and placebo group. The changes in Ct value from baseline were significantly greater in clevudine group compared to placebo group in patients with hypertension, and patients who underwent randomization during the first 5 and 7 days after the onset of symptoms. All clinical efficacy outcomes had no significant difference between clevudine group and placebo group. Clevudine was well tolerated and there was no significant difference in safety profile between two treatment groups. Conclusions: This is the first clinical study to compare the antiviral efficacy and safety of clevudine to placebo in Korean COVID-19 patients with moderate severity. The study has demonstrated a possible favorable outcome for the reduction of SARS-CoV-2 replication, with acceptable safety profile, when COVID-19 patients were treated with clevudine. Further largescale clinical studies, preferably with various clinical endpoints and virus titer evaluation, are required to better understand the effectiveness of using clevudine in COVID-19 treatment. Considering recent trend in clinical development for antiviral drugs, we need to design a clinical study aiming for reducing clinical risk of COVID-19 in mild to moderate patients with at least one risk factor for serious illness.
References
Alam, Kabir, Comorbidities might be a risk factor for the incidence of COVID-19: Evidence from a web-based survey, Prev Med Rep
Arabi, Gordon, Derde, Lopinavir-ritonavir and hydroxychloroquine for critically ill patients with COVID-19: REMAP-CAP randomized controlled trial, Intensive Care Med
Breining, Frølund, Højen, Camostat mesylate against SARS-CoV-2 and COVID-19-Rationale, dosing and safety, Basic Clin Pharmacol Toxicol
Chhetri, Brahman, Molecular Docking Study of Some Nucleoside Analogs against Main Protease of SARS-CoV-2
Furuta, Komeno, Nakamura, Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase, Proc Jpn Acad Ser B Phys Biol Sci
Gentilotti, Savoldi, Compri, Assessment of COVID-19 progression on day 5 from symptoms onset, BMC Infect Dis
Gurung, Ali, Lee, Farah, Km, The potential of Paritaprevir and Emetine as inhibitors of SARS-CoV-2 RdRp, Saudi J Biol Sci
Halford, Wan, Dragoni, SPIKE-1: A Randomised Phase II/III trial in a community setting, assessing use of camostat in reducing the clinical progression of COVID-19 by blocking SARS-CoV-2 Spike protein-initiated membrane fusion, Trials
Hui, Lau, Clevudine for the treatment of chronic hepatitis B virus infection, Expert Opin Investig Drugs
Jang, Rhee, Wi, Jung, Viral kinetics of SARS-CoV-2 over the preclinical, clinical, and postclinical period, Int J Infect Dis
Ji, Huh, Kang, Effect of Underlying Comorbidities on the Infection and Severity of COVID-19 in Korea: a Nationwide Case-Control Study, J Korean Med Sci
Jones, Murakami, Delaney, Furman, Hu, Noncompetitive inhibition of hepatitis B virus reverse transcriptase protein priming and DNA synthesis by the nucleoside analog clevudine, Antimicrob Agents Chemother
Kokic, Hillen, Tegunov, Mechanism of SARS-CoV-2 polymerase stalling by remdesivir, Nat Commun
Lee, Hong, Kim, Lee, Lee, Clinical Course of Asymptomatic and Mildly Symptomatic Patients with Coronavirus Disease Admitted to Community Treatment Centers, South Korea, Emerg Infect Dis
Lescure, Bouadma, Nguyen, Clinical and virological data of the first cases of COVID-19 in Europe: a case series, Lancet Infect Dis
Malik, Gupta, Zhong, Rasmussen, Manautou et al., Emerging Therapeutic Modalities against COVID-19, Pharmaceuticals
Mody, Ho, Wills, Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents, Commun Biol
My, Hieu, Hai, Study on SARS-CoV-2 inhibition of some potential drugs using molecular docking simulation, Vietnam Journal of Chemistry
Navar, Purinton, Hou, Taylor, Peterson, The impact of race and ethnicity on outcomes in 19,584 adults hospitalized with COVID-19, PLoS One
Rao, Manissero, Steele, Pareja, A Systematic Review of the Clinical Utility of Cycle Threshold Values in the Context of COVID-19, Infect Dis Ther
Sadie, Sarah, Rahayma, Jennifer, Michael, The Impact of Age, Sex, and Race on the Association of Risk Factors and Mortality in COVID-19 Patients, Journal of Infectious Diseases and Epidemiology
Semenzato, Botton, Drouin, Antihypertensive Drugs and COVID-19 Risk: A Cohort Study of 2 Million Hypertensive Patients, Hypertension
Sisay, 3CL(pro) inhibitors as a potential therapeutic option for COVID-19: Available evidence and ongoing clinical trials, Pharmacol Res
Suh, Kim, Heo, Clinical Characteristics of COVID-19: Clinical Dynamics of Mild Severe Acute Respiratory Syndrome Coronavirus 2 Infection Detected by Early Active Surveillance, J Korean Med Sci
Tom, Mina, To Interpret the SARS-CoV-2 Test, Consider the Cycle Threshold Value, Clin Infect Dis
Udwadia, Singh, Barkate, Efficacy and safety of favipiravir, an oral RNAdependent RNA polymerase inhibitor, in mild-to-moderate COVID-19: A randomized, comparative, open-label, multicenter, phase 3 clinical trial, Int J Infect Dis
Wang, Zhang, Du, Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial, Lancet
Wing, Davenne, Wettengel, A dual role for SAMHD1 in regulating HBV cccDNA and RT-dependent particle genesis, Life Sci Alliance
Yan, Huang, Xu, Antihypertensive drugs are associated with reduced fatal outcomes and improved clinical characteristics in elderly COVID-19 patients, Cell Discov
Yang, Zheng, Gou, Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis, Int J Infect Dis
Zeng, Ma, Zhou, Spectrum and Clinical Characteristics of Symptomatic and Asymptomatic Coronavirus Disease 2019 (COVID-19) With and Without Pneumonia, Front Med
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1101/2021.12.09.21267566",
"URL": "http://dx.doi.org/10.1101/2021.12.09.21267566",
"abstract": "<jats:p>Background: Clevudine, an antiviral drug for chronic hepatitis B virus infection, is expected to inhibit the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus. Therefore, we conducted a prospective, single-blind, proof of concept clinical study to examine the antiviral efficacy and safety of clevudine compared to placebo in Korean corona virus disease 19 (COVID-19) patients with moderate severity.\nMethods: Adults with confirmed SARS-CoV-2 infection and symptom onset within 7 days were randomized 2:1 to 120 mg clevudine or placebo to receive one of treatments orally once-daily for 14 days. Antiviral efficacy outcomes were the proportion of patients with real-time reverse transcription polymerase chain reaction (RT-PCR) negative result for SARS-CoV-2 infection and cycle threshold (Ct) value changes from baseline. Clinical efficacy outcomes included proportion of patients who showed improvement in lung involvement by imaging tests, proportion of patients with normal body temperature, proportion of patients with normal oxygen saturation, and the changes in C-reactive protein (CRP) from baseline. Safety outcomes included changes in clinical laboratory tests, vital signs measurement, and physical examination from baseline, and incidence of adverse events.\nResults: The proportion of patients with real-time RT-PCR negative test and Ct value changes showed no significant difference between clevudine group and placebo group. The changes in Ct value from baseline were significantly greater in clevudine group compared to placebo group in patients with hypertension, and patients who underwent randomization during the first 5 and 7 days after the onset of symptoms. All clinical efficacy outcomes had no significant difference between clevudine group and placebo group. Clevudine was well tolerated and there was no significant difference in safety profile between two treatment groups. \nConclusions: This is the first clinical study to compare the antiviral efficacy and safety of clevudine to placebo in Korean COVID-19 patients with moderate severity. The study has demonstrated a possible favorable outcome for the reduction of SARS-CoV-2 replication, with acceptable safety profile, when COVID-19 patients were treated with clevudine. Further large-scale clinical studies, preferably with various clinical endpoints and virus titer evaluation, are required to better understand the effectiveness of using clevudine in COVID-19 treatment. Considering recent trend in clinical development for antiviral drugs, we need to design a clinical study aiming for reducing clinical risk of COVID-19 in mild to moderate patients with at least one risk factor for serious illness.</jats:p>",
"accepted": {
"date-parts": [
[
2021,
12,
14
]
]
},
"author": [
{
"ORCID": "http://orcid.org/0000-0002-0148-7194",
"affiliation": [],
"authenticated-orcid": false,
"family": "Song",
"given": "Joon-Young",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0003-1142-5488",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Yeon-Sook",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2744-1159",
"affiliation": [],
"authenticated-orcid": false,
"family": "Eom",
"given": "Joong-Sik",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4306-1597",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Jin-Yong",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7862-5519",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Jin-Soo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7041-065X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Jacob",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-5874-4764",
"affiliation": [],
"authenticated-orcid": false,
"family": "Choi",
"given": "Won-Suk",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6548-1939",
"affiliation": [],
"authenticated-orcid": false,
"family": "Heo",
"given": "Jung-Yeon",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4792-0456",
"affiliation": [],
"authenticated-orcid": false,
"family": "Sohn",
"given": "Jang-Wook",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-7668-6158",
"affiliation": [],
"authenticated-orcid": false,
"family": "Lee",
"given": "Ki-Deok",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-4515-521X",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cho",
"given": "Donghui",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-5789-2092",
"affiliation": [],
"authenticated-orcid": false,
"family": "Cho",
"given": "IlYoung",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-4546-3880",
"affiliation": [],
"authenticated-orcid": false,
"family": "Kim",
"given": "Woo-Joo",
"sequence": "additional"
}
],
"container-title": [],
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T00:30:12Z",
"timestamp": 1639528212000
},
"deposited": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T00:30:13Z",
"timestamp": 1639528213000
},
"group-title": "Infectious Diseases (except HIV/AIDS)",
"indexed": {
"date-parts": [
[
2021,
12,
15
]
],
"date-time": "2021-12-15T06:47:57Z",
"timestamp": 1639550877792
},
"institution": [
{
"name": "medRxiv"
}
],
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2021,
12,
14
]
]
},
"link": [
{
"URL": "https://syndication.highwire.org/content/doi/10.1101/2021.12.09.21267566",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "246",
"original-title": [],
"posted": {
"date-parts": [
[
2021,
12,
14
]
]
},
"prefix": "10.1101",
"published": {
"date-parts": [
[
2021,
12,
14
]
]
},
"publisher": "Cold Spring Harbor Laboratory",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [],
"short-title": [],
"source": "Crossref",
"subtitle": [],
"subtype": "preprint",
"title": [
"Oral antiviral clevudine compared with placebo in Korean COVID-19 patients with moderate severity"
],
"type": "posted-content"
}