Dec 14 2021 |
et al., medRxiv, doi:10.1101/2021.12.09.21267566 | Oral antiviral clevudine compared with placebo in Korean COVID-19 patients with moderate severity |
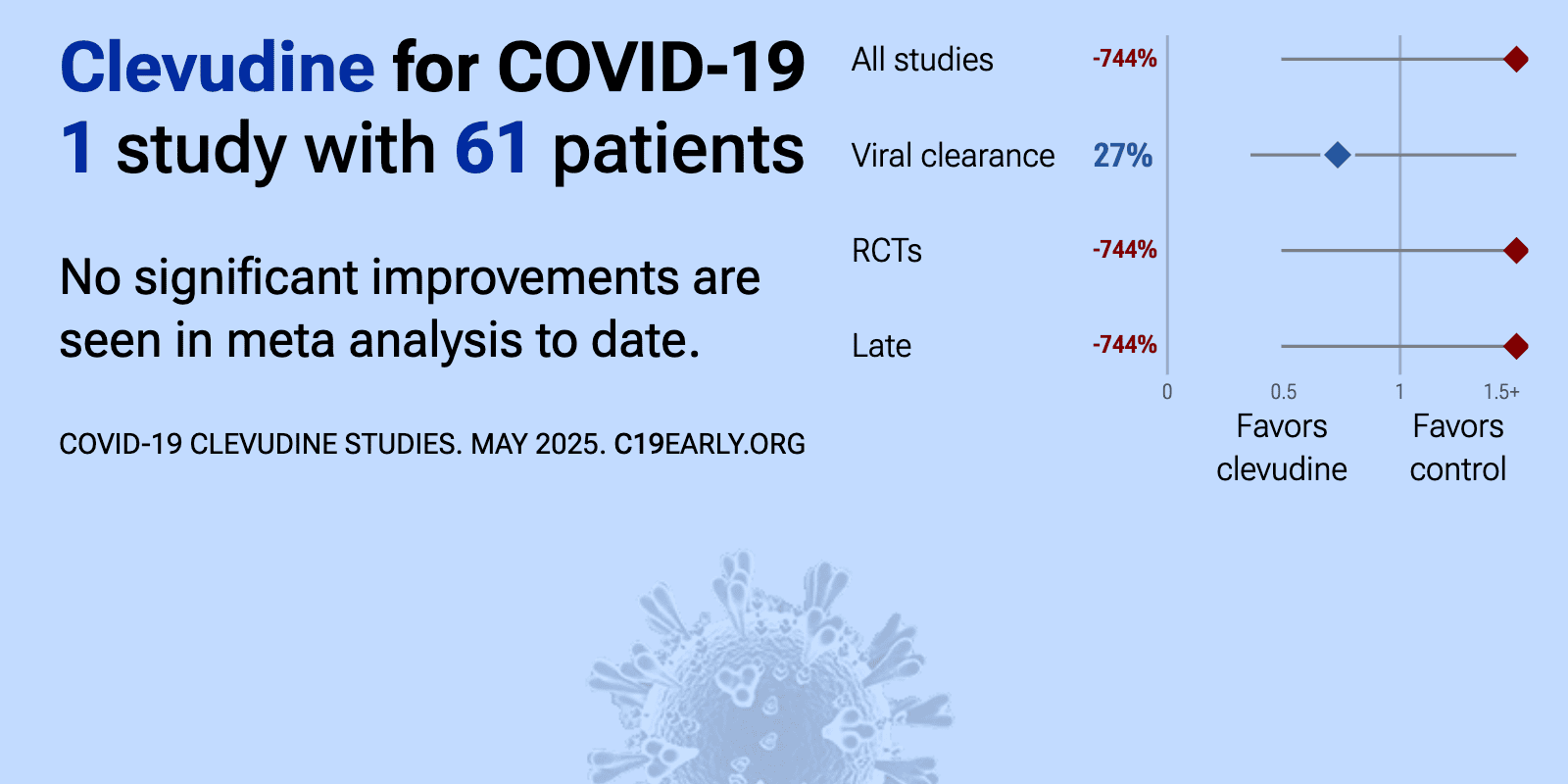
| 744% worse recovery (p=0.16) and 27% improved viral clearance (p=0.56). RCT 61 moderate COVID-19 patients in Korea showing no significant difference in viral clearance or clinical outcomes with clevudine compared to placebo. | ||
Jul 8 2021 |
et al., NCT04891302 | A Double Blind, Randomized, Placebo-controlled, Multi-center, Proof of Concept Phase 2 Study to Evaluate the Safety and Efficacy of Clevudine in Patients Diagnosed With Mild and Moderate COVID-19 |
| 104 patient clevudine late treatment RCT with results not reported over 4 years after completion. | ||