Standardized Brazilian green propolis extract (EPP-AF®) in COVID-19 outcomes: a randomized double-blind placebo-controlled trial
et al., Scientific Reports, doi:10.1038/s41598-023-43764-w, BeeCovid2, NCT04800224, Oct 2023
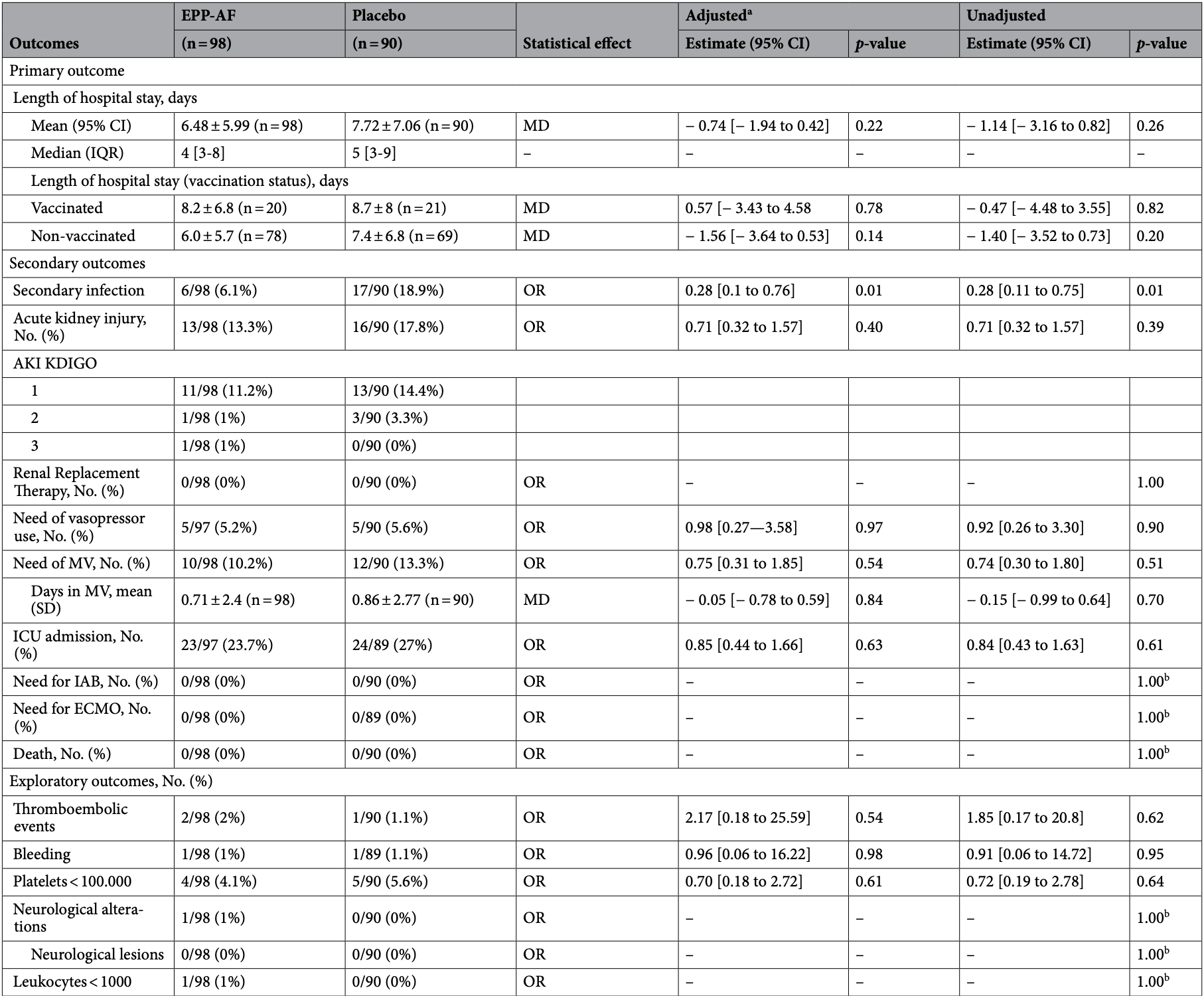
RCT 188 patients in Brazil, showing shorter hospitalization and improved outcomes with propolis, but without statistical significance. The incidence of secondary infections was significantly lower in the treatment group.
|
risk of mechanical ventilation, 22.4% lower, RR 0.78, p = 0.54, treatment 10 of 98 (10.2%), control 12 of 90 (13.3%), NNT 32, adjusted per study, odds ratio converted to relative risk.
|
|
risk of ICU admission, 11.4% lower, RR 0.89, p = 0.65, treatment 23 of 97 (23.7%), control 24 of 89 (27.0%), NNT 31, adjusted per study, odds ratio converted to relative risk.
|
|
hospitalization time, 16.1% lower, relative time 0.84, p = 0.19, treatment mean 6.48 (±5.99) n=98, control mean 7.72 (±7.06) n=90.
|
|
secondary infection, 67.6% lower, RR 0.32, p = 0.02, treatment 6 of 98 (6.1%), control 17 of 90 (18.9%), NNT 7.8, adjusted per study, odds ratio converted to relative risk.
|
|
AKI, 25.1% lower, RR 0.75, p = 0.41, treatment 13 of 98 (13.3%), control 16 of 90 (17.8%), NNT 22, adjusted per study, odds ratio converted to relative risk.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Silveira et al., 27 Oct 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Brazil, peer-reviewed, 24 authors, study period April 2021 - August 2021, trial NCT04800224 (history) (BeeCovid2).
Contact: marceloadsilveira@gmail.com.
Standardized Brazilian green propolis extract (EPP-AF®) in COVID-19 outcomes: a randomized double-blind placebo-controlled trial
Scientific Reports, doi:10.1038/s41598-023-43764-w
SARS-CoV-2 and its different variants caused a "wave and wave" pandemic pattern. During the first wave we demonstrated that standardized Brazilian green propolis extract (EPP-AF®) reduces length of hospital stay in adult patients with COVID-19. Afterwards, we decided to evaluate the impact of EPP-AF in hospitalized patients during the third wave of the pandemic. BeeCovid2 was a randomized, double-blind, placebo-controlled clinical trial in hospitalized COVID-19 adult patients. Patients were allocated to receive an oral dose of 900 mg/day of EPP-AF® or placebo for 10 days. The primary outcome was length of hospital stay. Secondary outcomes included safety, secondary infection rate, duration of oxygen therapy dependency, acute kidney injury and need for intensive care. Patients were followed up for 28 days after admission. We enrolled 188 patients; 98 were assigned to the propolis group and 90 to the placebo group. The post-intervention length of hospital stay was of 6.5 ± 6.0 days in the propolis group versus 7.7 ± 7.1 days in the control group (95% CI -0.74 [-1.94 to 0.42]; p = 0.22). Propolis did not have significant impact on the need for oxygen supplementation or frequency of AKI. There was a significant difference in the incidence of secondary infection between groups, with 6.1% in the propolis group versus 18.9% in the control group (95% CI -0.28 [0.1-0.76], p = 0.01). The use of EPP-AF was considered safe and associated with a decrease in secondary infections. The drug was not associated with a significant reduction in length of hospital stay.
ClinicalTrials.gov (NCT04800224). Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been a significant concern regarding its global impact on healthcare settings 1 . After the viral replication phase, there is an enormous immunological and inflammatory challenge since both innate and adaptive immunity may be disorderly activated by SARS-CoV-2 infection 2 . The magnitude of OPEN
Author contributions
Competing interests The authors declare no competing interests.
Additional information
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1038/ s41598-023-43764-w. Correspondence and requests for materials should be addressed to M.A.D.S. Reprints and permissions information is available at www.nature.com/reprints. Publisher's note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ahmadian, Covid-19 and kidney injury: Pathophysiology and molecular mechanisms, Rev. Med. Virol, doi:10.1002/rmv.2176
Anka, Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management, Scand. J. Immunol, doi:10.1111/sji.12998
Bahceci, Secondary bacterial infection rates among patients With COVID-19, Cureus, doi:10.7759/cureus.22363
Berretta, Nascimento, Bueno, Vaz, Marchetti, Propolis standardized extract (EPP-AF®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds, Int. J. Biol. Sci, doi:10.7150/ijbs.3641
Berretta, Silveira, Cóndor Capcha, De Jong, Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19, Biomed. Pharmacother, doi:10.1016/j.biopha.2020.110622
Bojko, Drug dosing using estimated glomerular filtration rate: Misclassification due to metamizole interference in a creatinine assay, Ann. Clin. Biochem, doi:10.1177/00045632211020029
Chilamakuri, Agarwal, COVID-19: Characteristics and therapeutics, Cells, doi:10.3390/cells10020206
Chong, State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia, Infection, doi:10.1007/s15010-021-01602-z
Cusinato, Evaluation of potential herbal-drug interactions of a standardized propolis extract (EPP-AF®) using an in vivo cocktail approach, J. Ethnopharmacol, doi:10.1016/j.jep.2019.112174
Da, Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer, Anal Cell Pathol (Amst), doi:10.1155/2019/1907698
De Bruyn, Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation, BMC Infect Dis, doi:10.1186/s12879-022-07192-x
Duarte Silveira, Effects of standardized Brazilian green propolis extract (EPP-AF®) on inflammation in haemodialysis patients: A clinical trial, Int. J. Nephrol, doi:10.1155/2022/1035475
Fajgenbaum, June, Cytokine storm, N. Engl. J. Med, doi:10.1056/NEJMra2026131
Giamarellos-Bourboulis, Complex immune dysregulation in COVID-19 patients with severe respiratory failure, Cell Host. Microbe, doi:10.1016/j.chom.2020.04.009
Grasselli, Cattaneo, Florio, Secondary infections in critically ill patients with COVID-19, Crit. Care, doi:10.1186/s13054-021-03672-9
Guler, Tatar, Yildiz, Belduz, Kolayli, Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study, Arch. Microbiol, doi:10.1007/s00203-021-02351-1
Hoffmann, SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor, Cell, doi:10.1016/j.cell.2020.02.052
Machado, Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity, Evid. Based Complement. Alternat. Med, doi:10.1155/2012/157652
Marquiafável, Development and characterization of a novel standardized propolis dry extract obtained by factorial design with high artepillin C content, J. Pharm. Technol. Drug Res, doi:10.7243/2050-120X-4-1
Maruta, He, PAK1-blockers: Potential therapeutics against COVID-19, Med. Drug. Discov, doi:10.1016/j.medidd.2020.100039
Melsen, Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies, Lancet Infect. Dis, doi:10.1016/S1473-3099(13)70081-1
Miethke-Morais, COVID-19-related hospital cost-outcome analysis: The impact of clinical and demographic factors, Braz. J. Infect. Dis, doi:10.1016/j.bjid.2021.101609
Minihan, Association between tocilizumab treatment of hyperinflammatory patients with COVID-19 in a critical care setting and elevated incidence of hospital-acquired bacterial and invasive fungal infections, J Hosp Infect, doi:10.1016/j.jhin.2022.04.007
Nogueira, Silva, Moura, Duarte Silveira, Moura-Neto, Acute kidney injury and electrolyte disorders in COVID-19, World J. Virol, doi:10.5501/wjv.v11.i5.283
Rocha, Evaluation of a propolis water extract using a reliable RP-HPLC methodology and in vitro and in vivo efficacy and safety characterisation, Evid. Based Complement. Alternat. Med, doi:10.1155/2013/670451
Silveira, Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: A randomized, double-blind, placebo-controlled trial, BMC Nephrol, doi:10.1186/s12882-019-1337-7
Silveira, Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial, Biomed. Pharmacother, doi:10.1016/j.biopha.2021.111526
Silveira, Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis, Sci. Rep, doi:10.1038/s41598-021-85124-6
Silveira, The use of standardized Brazilian green propolis extract (EPP-AF) as an adjunct treatment for hospitalized COVID-19 patients (BeeCovid2): A structured summary of a study protocol for a randomized controlled trial, Trials, doi:10.1186/s13063-022-06176-1
Woisky, Salatino, Analysis of propolis: Some parameters and procedures for chemical quality control, J. Apic. Res, doi:10.1080/00218839.1998.11100961
Wu, Acute kidney injury associated with remdesivir: A comprehensive pharmacovigilance analysis of COVID-19 reports in FAERS, Front. Pharmacol, doi:10.3389/fphar.2022.692828
DOI record:
{
"DOI": "10.1038/s41598-023-43764-w",
"ISSN": [
"2045-2322"
],
"URL": "http://dx.doi.org/10.1038/s41598-023-43764-w",
"abstract": "<jats:title>Abstract</jats:title><jats:p>SARS-CoV-2 and its different variants caused a “wave and wave” pandemic pattern. During the first wave we demonstrated that standardized Brazilian green propolis extract (EPP-AF®) reduces length of hospital stay in adult patients with COVID-19. Afterwards, we decided to evaluate the impact of EPP-AF in hospitalized patients during the third wave of the pandemic. BeeCovid2 was a randomized, double-blind, placebo-controlled clinical trial in hospitalized COVID-19 adult patients. Patients were allocated to receive an oral dose of 900 mg/day of EPP-AF® or placebo for 10 days. The primary outcome was length of hospital stay. Secondary outcomes included safety, secondary infection rate, duration of oxygen therapy dependency, acute kidney injury and need for intensive care. Patients were followed up for 28 days after admission. We enrolled 188 patients; 98 were assigned to the propolis group and 90 to the placebo group. The post-intervention length of hospital stay was of 6.5 ± 6.0 days in the propolis group versus 7.7 ± 7.1 days in the control group (95% CI − 0.74 [− 1.94 to 0.42]; <jats:italic>p</jats:italic> = 0.22). Propolis did not have significant impact on the need for oxygen supplementation or frequency of AKI. There was a significant difference in the incidence of secondary infection between groups, with 6.1% in the propolis group versus 18.9% in the control group (95% CI − 0.28 [0.1–0.76], <jats:italic>p</jats:italic> = 0.01). The use of EPP-AF was considered safe and associated with a decrease in secondary infections. The drug was not associated with a significant reduction in length of hospital stay. ClinicalTrials.gov (NCT04800224).</jats:p>",
"alternative-id": [
"43764"
],
"article-number": "18405",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "8 March 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "28 September 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "27 October 2023"
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Silveira",
"given": "Marcelo Augusto Duarte",
"sequence": "first"
},
{
"affiliation": [],
"family": "Menezes",
"given": "Matheus de Alencar",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Souza",
"given": "Sergio Pinto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galvão",
"given": "Erica Batista dos Santos",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berretta",
"given": "Andresa Aparecida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caldas",
"given": "Juliana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teixeira",
"given": "Maurício Brito",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gomes",
"given": "Marcel Miranda Dantas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Damiani",
"given": "Lucas Petri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bahiense",
"given": "Bruno Andrade",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cabral",
"given": "Julia Barros",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Oliveira",
"given": "Cicero Wandson Luiz Macedo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mascarenhas",
"given": "Talita Rocha",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pinheiro",
"given": "Priscila Carvalho Guedes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Alves",
"given": "Milena Souza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "de Melo",
"given": "Rodrigo Morel Vieira",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leite",
"given": "Flávia Mendes",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nonaka",
"given": "Carolina Kymie Vasques",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Souza",
"given": "Bruno Solano de Freitas",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baptista",
"given": "Nathália Ursoli",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Teles",
"given": "Flávio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "da Guarda",
"given": "Suzete Farias",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mendes",
"given": "Ana Verena Almeida",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Passos",
"given": "Rogério da Hora",
"sequence": "additional"
}
],
"container-title": "Scientific Reports",
"container-title-short": "Sci Rep",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2023,
10,
27
]
],
"date-time": "2023-10-27T10:02:58Z",
"timestamp": 1698400978000
},
"deposited": {
"date-parts": [
[
2023,
10,
27
]
],
"date-time": "2023-10-27T10:04:21Z",
"timestamp": 1698401061000
},
"indexed": {
"date-parts": [
[
2023,
10,
28
]
],
"date-time": "2023-10-28T17:11:03Z",
"timestamp": 1698513063218
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2023,
10,
27
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
27
]
],
"date-time": "2023-10-27T00:00:00Z",
"timestamp": 1698364800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
10,
27
]
],
"date-time": "2023-10-27T00:00:00Z",
"timestamp": 1698364800000
}
}
],
"link": [
{
"URL": "https://www.nature.com/articles/s41598-023-43764-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-43764-w",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://www.nature.com/articles/s41598-023-43764-w.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1038",
"published": {
"date-parts": [
[
2023,
10,
27
]
]
},
"published-online": {
"date-parts": [
[
2023,
10,
27
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.3390/cells10020206",
"author": "R Chilamakuri",
"doi-asserted-by": "publisher",
"first-page": "206",
"issue": "2",
"journal-title": "Cells.",
"key": "43764_CR1",
"unstructured": "Chilamakuri, R. & Agarwal, S. COVID-19: Characteristics and therapeutics. Cells. 10(2), 206. https://doi.org/10.3390/cells10020206 (2021).",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1111/sji.12998",
"author": "AU Anka",
"doi-asserted-by": "publisher",
"first-page": "e12998",
"issue": "4",
"journal-title": "Scand. J. Immunol.",
"key": "43764_CR2",
"unstructured": "Anka, A. U. et al. Coronavirus disease 2019 (COVID-19): An overview of the immunopathology, serological diagnosis and management. Scand. J. Immunol. 93(4), e12998. https://doi.org/10.1111/sji.12998 (2021).",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1007/s15010-021-01602-z",
"author": "WH Chong",
"doi-asserted-by": "publisher",
"first-page": "591",
"issue": "4",
"journal-title": "Infection.",
"key": "43764_CR3",
"unstructured": "Chong, W. H. et al. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 49(4), 591–605. https://doi.org/10.1007/s15010-021-01602-z (2021).",
"volume": "49",
"year": "2021"
},
{
"DOI": "10.1056/NEJMra2026131",
"author": "DC Fajgenbaum",
"doi-asserted-by": "publisher",
"first-page": "2255",
"issue": "23",
"journal-title": "N. Engl. J. Med.",
"key": "43764_CR4",
"unstructured": "Fajgenbaum, D. C. & June, C. H. Cytokine storm. N. Engl. J. Med. 383(23), 2255–2273. https://doi.org/10.1056/NEJMra2026131 (2020).",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1155/2012/157652",
"author": "JL Machado",
"doi-asserted-by": "publisher",
"first-page": "157652",
"journal-title": "Evid. Based Complement. Alternat. Med.",
"key": "43764_CR5",
"unstructured": "Machado, J. L. et al. Brazilian green propolis: Anti-inflammatory property by an immunomodulatory activity. Evid. Based Complement. Alternat. Med. 2012, 157652. https://doi.org/10.1155/2012/157652 (2012).",
"volume": "2012",
"year": "2012"
},
{
"DOI": "10.1016/j.biopha.2020.110622",
"author": "AA Berretta",
"doi-asserted-by": "publisher",
"first-page": "110622",
"journal-title": "Biomed. Pharmacother.",
"key": "43764_CR6",
"unstructured": "Berretta, A. A., Silveira, M. A. D., Cóndor Capcha, J. M. & De Jong, D. Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease: Running title: Propolis against SARS-CoV-2 infection and COVID-19. Biomed. Pharmacother. 131, 110622. https://doi.org/10.1016/j.biopha.2020.110622 (2020).",
"volume": "131",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2020.02.052",
"author": "M Hoffmann",
"doi-asserted-by": "publisher",
"first-page": "271",
"issue": "2",
"journal-title": "Cell.",
"key": "43764_CR7",
"unstructured": "Hoffmann, M. et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181(2), 271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052 (2020).",
"volume": "181",
"year": "2020"
},
{
"DOI": "10.1007/s00203-021-02351-1",
"author": "HI Guler",
"doi-asserted-by": "publisher",
"first-page": "3557",
"issue": "6",
"journal-title": "Arch. Microbiol.",
"key": "43764_CR8",
"unstructured": "Guler, H. I., Tatar, G., Yildiz, O., Belduz, A. O. & Kolayli, S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by molecular docking study. Arch. Microbiol. 203(6), 3557–3564. https://doi.org/10.1007/s00203-021-02351-1 (2021).",
"volume": "203",
"year": "2021"
},
{
"DOI": "10.1016/j.medidd.2020.100039",
"author": "H Maruta",
"doi-asserted-by": "publisher",
"first-page": "100039",
"journal-title": "Med. Drug. Discov.",
"key": "43764_CR9",
"unstructured": "Maruta, H. & He, H. PAK1-blockers: Potential therapeutics against COVID-19. Med. Drug. Discov. 6, 100039. https://doi.org/10.1016/j.medidd.2020.100039 (2020).",
"volume": "6",
"year": "2020"
},
{
"DOI": "10.1155/2019/1907698",
"author": "J Da",
"doi-asserted-by": "publisher",
"first-page": "1907698",
"journal-title": "Anal Cell Pathol (Amst).",
"key": "43764_CR10",
"unstructured": "Da, J. et al. Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer. Anal Cell Pathol (Amst). 2019, 1907698. https://doi.org/10.1155/2019/1907698 (2019).",
"volume": "2019",
"year": "2019"
},
{
"DOI": "10.1016/j.biopha.2021.111526",
"author": "MAD Silveira",
"doi-asserted-by": "publisher",
"first-page": "111526",
"journal-title": "Biomed. Pharmacother.",
"key": "43764_CR11",
"unstructured": "Silveira, M. A. D. et al. Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: A randomized, controlled clinical trial. Biomed. Pharmacother. 138, 111526. https://doi.org/10.1016/j.biopha.2021.111526 (2021).",
"volume": "138",
"year": "2021"
},
{
"DOI": "10.1186/s13063-022-06176-1",
"author": "MAD Silveira",
"doi-asserted-by": "publisher",
"first-page": "255",
"issue": "1",
"journal-title": "Trials.",
"key": "43764_CR12",
"unstructured": "Silveira, M. A. D. et al. The use of standardized Brazilian green propolis extract (EPP-AF) as an adjunct treatment for hospitalized COVID-19 patients (BeeCovid2): A structured summary of a study protocol for a randomized controlled trial. Trials. 23(1), 255. https://doi.org/10.1186/s13063-022-06176-1 (2022).",
"volume": "23",
"year": "2022"
},
{
"DOI": "10.7150/ijbs.3641",
"author": "AA Berretta",
"doi-asserted-by": "publisher",
"first-page": "512",
"issue": "4",
"journal-title": "Int. J. Biol. Sci.",
"key": "43764_CR13",
"unstructured": "Berretta, A. A., Nascimento, A. P., Bueno, P. C., Vaz, M. M. & Marchetti, J. M. Propolis standardized extract (EPP-AF®), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int. J. Biol. Sci. 8(4), 512–521. https://doi.org/10.7150/ijbs.3641 (2012).",
"volume": "8",
"year": "2012"
},
{
"DOI": "10.7243/2050-120X-4-1",
"author": "FS Marquiafável",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "J. Pharm. Technol. Drug Res.",
"key": "43764_CR14",
"unstructured": "Marquiafável, F. S. et al. Development and characterization of a novel standardized propolis dry extract obtained by factorial design with high artepillin C content. J. Pharm. Technol. Drug Res. 4(1), 1–13. https://doi.org/10.7243/2050-120X-4-1 (2015).",
"volume": "4",
"year": "2015"
},
{
"DOI": "10.1080/00218839.1998.11100961",
"author": "RG Woisky",
"doi-asserted-by": "publisher",
"first-page": "99",
"issue": "2",
"journal-title": "J. Apic. Res.",
"key": "43764_CR15",
"unstructured": "Woisky, R. G. & Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 37(2), 99–105. https://doi.org/10.1080/00218839.1998.11100961 (1998).",
"volume": "37",
"year": "1998"
},
{
"DOI": "10.1155/2013/670451",
"author": "BA Rocha",
"doi-asserted-by": "publisher",
"first-page": "670451",
"journal-title": "Evid. Based Complement. Alternat. Med.",
"key": "43764_CR16",
"unstructured": "Rocha, B. A. et al. Evaluation of a propolis water extract using a reliable RP-HPLC methodology and in vitro and in vivo efficacy and safety characterisation. Evid. Based Complement. Alternat. Med. 2013, 670451. https://doi.org/10.1155/2013/670451 (2013).",
"volume": "2013",
"year": "2013"
},
{
"DOI": "10.1186/s12882-019-1337-7",
"author": "MAD Silveira",
"doi-asserted-by": "publisher",
"first-page": "140",
"issue": "1",
"journal-title": "BMC Nephrol.",
"key": "43764_CR17",
"unstructured": "Silveira, M. A. D. et al. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: A randomized, double-blind, placebo-controlled trial. BMC Nephrol. 20(1), 140. https://doi.org/10.1186/s12882-019-1337-7 (2019).",
"volume": "20",
"year": "2019"
},
{
"DOI": "10.1016/j.jep.2019.112174",
"author": "DAC Cusinato",
"doi-asserted-by": "publisher",
"first-page": "112174",
"journal-title": "J. Ethnopharmacol.",
"key": "43764_CR18",
"unstructured": "Cusinato, D. A. C. et al. Evaluation of potential herbal-drug interactions of a standardized propolis extract (EPP-AF®) using an in vivo cocktail approach. J. Ethnopharmacol. 245, 112174. https://doi.org/10.1016/j.jep.2019.112174 (2019).",
"volume": "245",
"year": "2019"
},
{
"DOI": "10.1016/j.bjid.2021.101609",
"author": "A Miethke-Morais",
"doi-asserted-by": "publisher",
"first-page": "101609",
"issue": "4",
"journal-title": "Braz. J. Infect. Dis.",
"key": "43764_CR19",
"unstructured": "Miethke-Morais, A. et al. COVID-19-related hospital cost-outcome analysis: The impact of clinical and demographic factors. Braz. J. Infect. Dis. 25(4), 101609. https://doi.org/10.1016/j.bjid.2021.101609 (2021).",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.1016/S1473-3099(13)70081-1",
"author": "WG Melsen",
"doi-asserted-by": "publisher",
"first-page": "665",
"issue": "8",
"journal-title": "Lancet Infect. Dis.",
"key": "43764_CR20",
"unstructured": "Melsen, W. G. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 13(8), 665–671. https://doi.org/10.1016/S1473-3099(13)70081-1 (2013).",
"volume": "13",
"year": "2013"
},
{
"DOI": "10.1016/j.chom.2020.04.009",
"author": "EJ Giamarellos-Bourboulis",
"doi-asserted-by": "publisher",
"first-page": "992",
"issue": "6",
"journal-title": "Cell Host. Microbe.",
"key": "43764_CR21",
"unstructured": "Giamarellos-Bourboulis, E. J. et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host. Microbe. 27(6), 992-1000.e3. https://doi.org/10.1016/j.chom.2020.04.009 (2020).",
"volume": "27",
"year": "2020"
},
{
"DOI": "10.1186/s12879-022-07192-x",
"author": "A De Bruyn",
"doi-asserted-by": "publisher",
"first-page": "207",
"issue": "1",
"journal-title": "BMC Infect Dis.",
"key": "43764_CR22",
"unstructured": "De Bruyn, A. et al. Secondary infection in COVID-19 critically ill patients: A retrospective single-center evaluation. BMC Infect Dis. 22(1), 207. https://doi.org/10.1186/s12879-022-07192-x (2022).",
"volume": "22",
"year": "2022"
},
{
"DOI": "10.1016/j.jhin.2022.04.007",
"author": "B Minihan",
"doi-asserted-by": "publisher",
"first-page": "29",
"journal-title": "J Hosp Infect.",
"key": "43764_CR23",
"unstructured": "Minihan, B. et al. Association between tocilizumab treatment of hyperinflammatory patients with COVID-19 in a critical care setting and elevated incidence of hospital-acquired bacterial and invasive fungal infections. J Hosp Infect. 126, 29–36. https://doi.org/10.1016/j.jhin.2022.04.007 (2022).",
"volume": "126",
"year": "2022"
},
{
"DOI": "10.1186/s13054-021-03672-9",
"author": "G Grasselli",
"doi-asserted-by": "publisher",
"first-page": "317",
"issue": "1",
"journal-title": "Crit. Care.",
"key": "43764_CR24",
"unstructured": "Grasselli, G., Cattaneo, E. & Florio, G. Secondary infections in critically ill patients with COVID-19. Crit. Care. 25(1), 317. https://doi.org/10.1186/s13054-021-03672-9 (2021).",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.7759/cureus.22363",
"author": "I Bahceci",
"doi-asserted-by": "publisher",
"first-page": "e22363",
"issue": "2",
"journal-title": "Cureus",
"key": "43764_CR25",
"unstructured": "Bahceci, I. et al. Secondary bacterial infection rates among patients With COVID-19. Cureus 14(2), e22363. https://doi.org/10.7759/cureus.22363 (2022).",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1155/2022/1035475",
"author": "MA Duarte Silveira",
"doi-asserted-by": "publisher",
"first-page": "1035475",
"journal-title": "Int. J. Nephrol.",
"key": "43764_CR26",
"unstructured": "Duarte Silveira, M. A. et al. Effects of standardized Brazilian green propolis extract (EPP-AF®) on inflammation in haemodialysis patients: A clinical trial. Int. J. Nephrol. 2022, 1035475. https://doi.org/10.1155/2022/1035475 (2022).",
"volume": "2022",
"year": "2022"
},
{
"DOI": "10.5501/wjv.v11.i5.283",
"author": "GM Nogueira",
"doi-asserted-by": "publisher",
"first-page": "283",
"issue": "5",
"journal-title": "World J. Virol.",
"key": "43764_CR27",
"unstructured": "Nogueira, G. M., Silva, N. L. O. R., Moura, A. F., Duarte Silveira, M. A. & Moura-Neto, J. A. Acute kidney injury and electrolyte disorders in COVID-19. World J. Virol. 11(5), 283–292. https://doi.org/10.5501/wjv.v11.i5.283 (2022).",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1002/rmv.2176",
"author": "E Ahmadian",
"doi-asserted-by": "publisher",
"first-page": "e2176",
"issue": "3",
"journal-title": "Rev. Med. Virol.",
"key": "43764_CR28",
"unstructured": "Ahmadian, E. et al. Covid-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 31(3), e2176. https://doi.org/10.1002/rmv.2176 (2021).",
"volume": "31",
"year": "2021"
},
{
"DOI": "10.3389/fphar.2022.692828",
"author": "B Wu",
"doi-asserted-by": "publisher",
"first-page": "692828",
"journal-title": "Front. Pharmacol.",
"key": "43764_CR29",
"unstructured": "Wu, B. et al. Acute kidney injury associated with remdesivir: A comprehensive pharmacovigilance analysis of COVID-19 reports in FAERS. Front. Pharmacol. 13, 692828. https://doi.org/10.3389/fphar.2022.692828 (2022).",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.1038/s41598-021-85124-6",
"author": "MAD Silveira",
"doi-asserted-by": "publisher",
"first-page": "5925",
"issue": "1",
"journal-title": "Sci. Rep.",
"key": "43764_CR30",
"unstructured": "Silveira, M. A. D. et al. Green propolis extract attenuates acute kidney injury and lung injury in a rat model of sepsis. Sci. Rep. 11(1), 5925. https://doi.org/10.1038/s41598-021-85124-6 (2021).",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1177/00045632211020029",
"author": "L Bojko",
"doi-asserted-by": "publisher",
"first-page": "474",
"issue": "5",
"journal-title": "Ann. Clin. Biochem.",
"key": "43764_CR31",
"unstructured": "Bojko, L. et al. Drug dosing using estimated glomerular filtration rate: Misclassification due to metamizole interference in a creatinine assay. Ann. Clin. Biochem. 58(5), 474–480. https://doi.org/10.1177/00045632211020029 (2021).",
"volume": "58",
"year": "2021"
}
],
"reference-count": 31,
"references-count": 31,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.nature.com/articles/s41598-023-43764-w"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Multidisciplinary"
],
"subtitle": [],
"title": "Standardized Brazilian green propolis extract (EPP-AF®) in COVID-19 outcomes: a randomized double-blind placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "13"
}