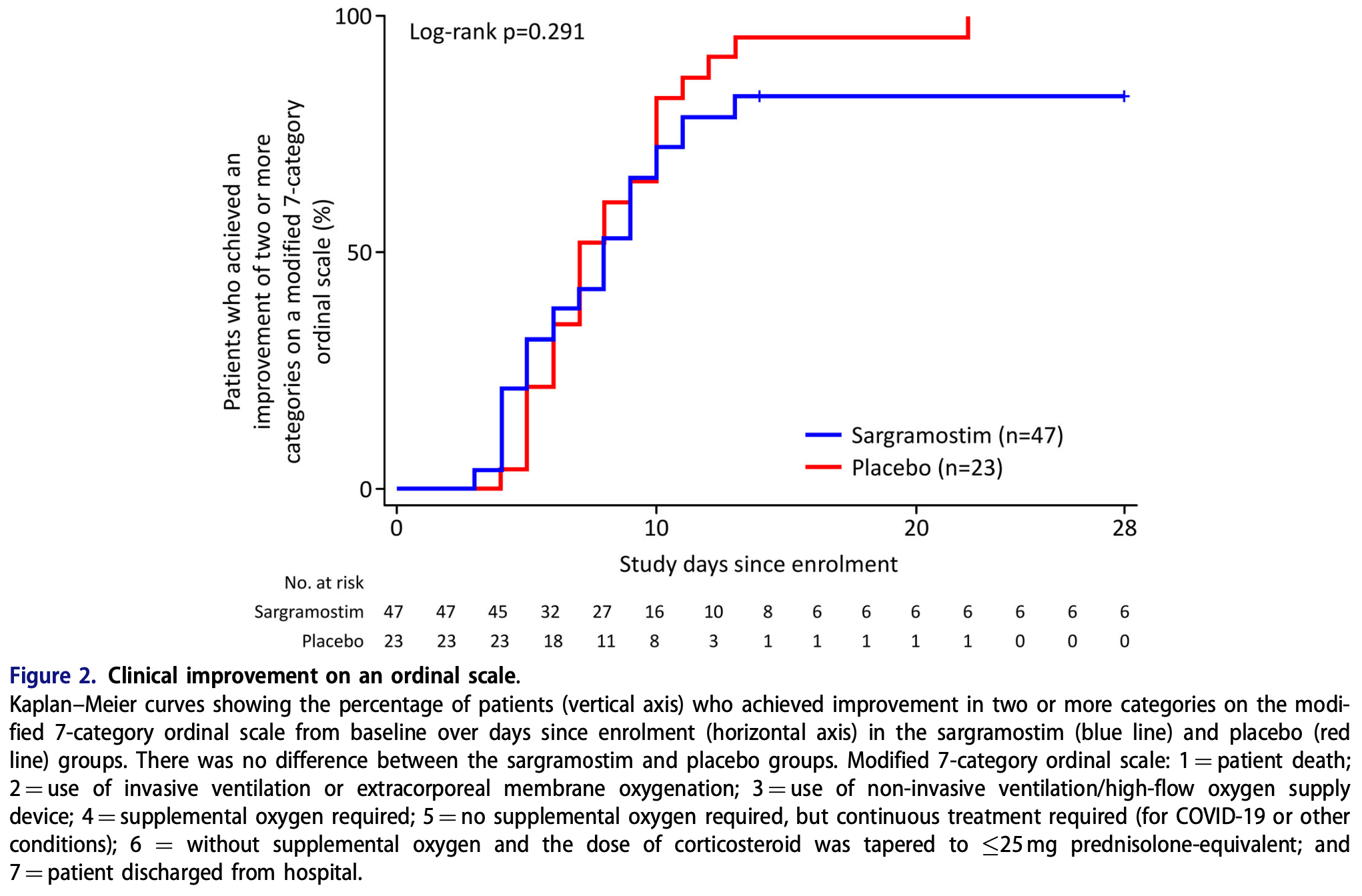
Short-term inhalation of sargramostim with concomitant high-dose steroids does not hasten recovery in moderate COVID-19 pneumonia: a double-blind, randomised, placebo-controlled trial
et al., Infectious Diseases, doi:10.1080/23744235.2023.2254380, NCT04642950, Sep 2023
RCT 70 hospitalized COVID-19 patients with moderate pneumonia in Japan showing no significant difference in time to clinical improvement with inhaled sargramostim (GM-CSF) vs. placebo. Concomitant corticosteroid dose was not standardized. In a post-hoc analysis of patients who received ≤500mg prednisolone-equivalent corticosteroids over 5 days, sargramostim improved oxygenation and clinical status compared to placebo. Higher corticosteroid doses were associated with delayed recovery. Authors hypothesize that high-dose corticosteroids may impair differentiation of monocytes into mature alveolar macrophages.
Standard of Care (SOC) for COVID-19 in the study country,
Japan, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of mechanical ventilation, 304.5% higher, RR 4.05, p = 0.54, treatment 2 of 44 (4.5%), control 0 of 23 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of no hospital discharge, 56.8% higher, RR 1.57, p = 1.00, treatment 3 of 44 (6.8%), control 1 of 23 (4.3%).
|
|
time to clinical improvement, 12.5% higher, relative time 1.12, p = 0.10, treatment 47, control 23.
|
|
hospitalization time, 10.0% higher, relative time 1.10, treatment 44, control 23.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Shimasaki et al., 20 Sep 2023, Double Blind Randomized Controlled Trial, placebo-controlled, Japan, peer-reviewed, mean age 54.7, 26 authors, study period 8 February, 2021 - 25 October, 2021, trial NCT04642950 (history).
Contact: niiiden2012@yahoo.co.jp.
Short-term inhalation of sargramostim with concomitant high-dose steroids does not hasten recovery in moderate COVID-19 pneumonia: a double-blind, randomised, placebo-controlled trial
Infectious Diseases, doi:10.1080/23744235.2023.2254380
Background: Granulocyte-macrophage colony stimulating factor (GM-CSF) inhalation may alleviate pulmonary inflammation caused by viral pneumonia. To investigate this, we evaluated its efficacy on COVID-19 pneumonia. Methods: This double-blind, randomised, placebo-controlled study (ClinicalTrials.gov: NCT04642950) evaluated patients in the first half of 2021 at seven Japanese hospitals. Hospitalised patients with COVID-19 pneumonia with moderate hypoxaemia inhaled sargramostim or placebo for 5 days. The primary endpoint was days to achieve a ! 2-category improvement from baseline on a modified 7-category ordinal scale. Secondary endpoints included degree of oxygenation, defined by amount of oxygen supply, and serum CCL17 level. Results: Seventy-five patients were randomly assigned in a 2:1 ratio to receive sargramostim or placebo, of which 47 and 23 were analysed, respectively. No difference was observed between groups regarding the primary endpoint (8.0 and 7.0 days for sargramostim and placebo, respectively) or in the secondary endpoints, except for CCL17. A post hoc sub-analysis indicated that endpoint assessments were influenced by concomitant corticosteroid therapy. When the cumulative corticosteroid dose was 500 mg during Days 1-5, recovery and oxygenation were faster in the sargramostim group than for placebo. Bolus dose corticosteroids were associated with temporarily impaired oxygenation and delayed clinical recovery. The increase in serum CCL17, a candidate prognostic factor, reflected improvement with sargramostim inhalation. The number of adverse events was similar between groups. Two serious adverse events were observed in the sargramostim group without causal relation.
Authors' contribution lying data reported in this manuscript. All authors had full The study was also sponsored by Nobelpharma, which was involved in the collection, analysis, and interpretation of the data; in the writing of the report, and in the decision to submit the paper for publication. The authors thank Dr Hannah Read, of Edanz (www.edanz.com) for medical writing assistance, which was funded by Nobelpharma, in accordance with Good Publication Practice guidelines (https://www.ismpp.org/gpp-2022). The authors also thank Dr Steven Holland and Dr Kiyoko Akagawa for critical review of this manuscript.
References
Bosteels, Van Damme, Leeuw, Loss of GM-CSF-dependent instruction of alveolar macrophages in COVID-19 provides a rationale for inhaled GM-CSF treatment, Cell Rep Med, doi:10.1016/j.xcrm.2022.100833
Carcaterra, Caruso, Alveolar epithelial cell type II as main target of SARS-CoV-2 virus and COVID-19 development via NF-Kb pathway deregulation: a physio-pathological theory, Med Hypotheses, doi:10.1016/j.mehy.2020.110412
Cereser, Girometti, Re, Inter-reader agreement of high-resolution computed tomography findings in patients with COVID-19 pneumonia: a multi-reader study, Radiol Med, doi:10.1007/s11547-020-01320-w
Chen, Lan, Ye, Cytokine storm: the primary determinant for the pathophysiological evolution of COVID-19 deterioration, Front Immunol, doi:10.3389/fimmu.2021.589095
Dorward, Russell, Um, Tissue-specific immunopathology in fatal COVID-19, Am J Respir Crit Care Med, doi:10.1164/rccm.202008-3265OC
Grant, Morales-Nebreda, Markov, Circuits between infected macrophages and T cells in SARS-CoV-2 pneumonia, Nature, doi:10.1038/s41586-020-03148-w
Halstead, Umstead, Davies, GM-CSF overexpression after influenza a virus infection prevents mortality and moderates M1-like airway monocyte/macrophage polarization, Respir Res, doi:10.1186/s12931-017-0708-5
Li, Hu, Song, High-dose but not low-dose corticosteroids potentially delay viral shedding of patients with COVID-19, Clin Infect Dis, doi:10.1093/cid/ciaa829
Li, Wu, Wu, The clinical and chest CT features associated with severe and critical COVID-19 pneumonia, Invest Radiol, doi:10.1097/RLI.0000000000000672
Min, Isa, Shuai, Cutting edge: granulocytemacrophage colony-stimulating factor is the major CD8þ T cell derived licensing factor for dendritic cell activation, J Immunol, doi:10.4049/jimmunol.0903873
Oda, Yamaura, Ishii, Quantitative evaluation of changes in three-dimensional CT density distributions in pulmonary alveolar proteinosis after GM-CSF inhalation, Respiration, doi:10.1159/000528038
Page, Goicochea, Matthews, Induction of alternatively activated macrophages enhances pathogenesis during severe acute respiratory syndrome coronavirus infection, J Virol, doi:10.1128/JVI.01689-12
Patroc Inio De Jesus, Silva, Aliyeva, Reactivation of SARS-CoV-2 after asymptomatic infection while on highdose corticosteroids. Case report, SN Compr Clin Med, doi:10.1007/s42399-020-00548-x
Potter, Boyd, Clarke, Recruiting the innate immune system with GM-CSF to fight viral diseases, including West Nile Virus encephalitis and COVID-19, Res, doi:10.12688/f1000research.23729.1
Recovery Collaborative, Higher dose corticosteroids in patients admitted to hospital with COVID-19 who are hypoxic but not requiring ventilatory support (RECOVERY): a randomised, controlled, open-label, platform trial, Lancet, doi:10.1016/S0140-6736(23)00510-X
Romanou, Koukaki, Chantziara, Dexamethasone in the treatment of COVID-19: primus inter pares?, J Pers Med, doi:10.3390/jpm11060556
R€ Osler, Lung epithelial GM-CSF improves host defense function and epithelial repair in influenza virus pneumonia-a new therapeutic strategy?, Mol Cell Pediatr, doi:10.1186/s40348-016-0055-5
Soffer, Morgenthau, Shimon, Artificial intelligence for interstitial lung disease analysis on chest computed tomography: a systematic review, Acad Radiol, doi:10.1016/j.acra.2021.05.014
Sugiyama, Predictive serum markers for severe COVID-19 illness, Translat Regulat Sci, doi:10.33611/trs.2021-018
Umbrello, Formenti, Nespoli, Effect of different corticosteroid regimens on the outcome of severe COVID-19-related acute respiratory failure. A retrospective analysis, J Clin Med, doi:10.3390/jcm10214847
Unkel, Hoegner, Clausen, Alveolar epithelial cells orchestrate DC function in murine viral pneumonia, J Clin Invest, doi:10.1172/JCI62139
Wang, Mao, Klein, Diverse functional autoantibodies in patients with COVID-19, Nature, doi:10.1038/s41586-021-03631-y
Wang, Yang, Chen, The proportion and effect of corticosteroid therapy in patients with COVID-19 infection: a systematic review and meta-analysis, PLOS One, doi:10.1371/journal.pone.0249481
Yang, Yang, Luo, Corticosteroid administration for viral pneumonia: COVID-19 and beyond, Clin Microbiol Infect, doi:10.1016/j.cmi.2020.06.020
Zhang, Tian, Feng, Judicious use of low-dosage corticosteroids for non-severe COVID-19: a case report, Open Med, doi:10.1515/med-2021-0250
DOI record:
{
"DOI": "10.1080/23744235.2023.2254380",
"ISSN": [
"2374-4235",
"2374-4243"
],
"URL": "http://dx.doi.org/10.1080/23744235.2023.2254380",
"alternative-id": [
"10.1080/23744235.2023.2254380"
],
"assertion": [
{
"label": "Peer Review Statement",
"name": "peerreview_statement",
"order": 1,
"value": "The publishing and review policy for this title is described in its Aims & Scope."
},
{
"URL": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=infd20",
"label": "Aim & Scope",
"name": "aims_and_scope_url",
"order": 2,
"value": "http://www.tandfonline.com/action/journalInformation?show=aimsScope&journalCode=infd20"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-05-10"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-08-25"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-09-20"
}
],
"author": [
{
"affiliation": [
{
"name": "Nobelpharma Co., Ltd, Chuo-ku, Japan"
}
],
"family": "Shimasaki",
"given": "Shigeki",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Kanagawa Cardiovascular and Respiratory Center, Yokohama, Japan"
}
],
"family": "Baba",
"given": "Tomohisa",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Kanagawa Cardiovascular and Respiratory Center, Yokohama, Japan"
}
],
"family": "Ogura",
"given": "Takashi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Saitama Red Cross Hospital, Saitama, Japan"
}
],
"family": "Akasaka",
"given": "Keiichi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Saitama Red Cross Hospital, Saitama, Japan"
}
],
"family": "Matsushima",
"given": "Hidekazu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, National Center for Global Health and Medicine, Shinjuku-ku, Japan"
}
],
"family": "Izumi",
"given": "Shinyu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, National Center for Global Health and Medicine, Shinjuku-ku, Japan"
}
],
"family": "Takasaki",
"given": "Jin",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, International University of Health and Welfare (IUHW), Chiba, Japan"
}
],
"family": "Tsushima",
"given": "Kenji",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, International University of Health and Welfare (IUHW), Chiba, Japan"
}
],
"family": "Kinouchi",
"given": "Toru",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Federation of National Public Service Personnel Mutual Aid Associations, Mishuku Hospital, Meguro-ku, Japan"
}
],
"family": "Kichikawa",
"given": "Yoshiko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Federation of National Public Service Personnel Mutual Aid Associations, Mishuku Hospital, Meguro-ku, Japan"
}
],
"family": "Awashima",
"given": "Maiko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Japanese Red Cross Medical Center, Shibuya-ku, Japan"
}
],
"family": "Izumo",
"given": "Takehiro",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Japanese Red Cross Medical Center, Shibuya-ku, Japan"
}
],
"family": "Awano",
"given": "Nobuyasu",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Pulmonary Medicine, Thoracic Center, St. Luke’s International Hospital, Chuo-ku, Japan"
}
],
"family": "Nishimura",
"given": "Naoki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Health Administration Center, Student Support and Health Administration Organization, Tokyo Medical and Dental University, Bunkyo-ku, Japan"
}
],
"family": "Tazawa",
"given": "Ryushi",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "National Center for Global Health and Medicine, Center for Clinical Sciences, Shinjuku-ku, Japan"
}
],
"family": "Mikami",
"given": "Ayako",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical and Translational Research Center, Niigata University Medical and Dental Hospital, Niigata, Japan"
}
],
"family": "Kitamura",
"given": "Nobutaka",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Respiratory Medicine, Kyorin University School of Medicine, Mitaka, Japan"
}
],
"family": "Ishii",
"given": "Haruyuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Radiology, St. Luke’s International Hospital, Chuo-ku, Japan"
}
],
"family": "Kurihara",
"given": "Yasuyuki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nobelpharma Co., Ltd, Chuo-ku, Japan"
}
],
"family": "Taniguchi",
"given": "Masaki",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nobelpharma Co., Ltd, Chuo-ku, Japan"
}
],
"family": "Aikawa",
"given": "Satoko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nobelpharma Co., Ltd, Chuo-ku, Japan"
}
],
"family": "Okada",
"given": "Mami",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nobelpharma Co., Ltd, Chuo-ku, Japan"
}
],
"family": "Morita",
"given": "Yusuke",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nobelpharma Co., Ltd, Chuo-ku, Japan"
}
],
"family": "Ishikawa",
"given": "Yuko",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Nobelpharma Co., Ltd, Chuo-ku, Japan"
}
],
"family": "Ohinata",
"given": "Akira",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Center for Medical Innovation, Division of Pioneering Advanced Therapeutics, Niigata University Medical Dental Hospital, Niigata, Japan"
}
],
"family": "Nakata",
"given": "Koh",
"sequence": "additional"
}
],
"container-title": "Infectious Diseases",
"container-title-short": "Infectious Diseases",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"www.tandfonline.com"
]
},
"created": {
"date-parts": [
[
2023,
9,
20
]
],
"date-time": "2023-09-20T16:48:13Z",
"timestamp": 1695228493000
},
"deposited": {
"date-parts": [
[
2024,
2,
15
]
],
"date-time": "2024-02-15T10:54:26Z",
"timestamp": 1707994466000
},
"indexed": {
"date-parts": [
[
2024,
2,
15
]
],
"date-time": "2024-02-15T11:11:46Z",
"timestamp": 1707995506388
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
9,
20
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2023,
12,
2
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
9,
20
]
],
"date-time": "2023-09-20T00:00:00Z",
"timestamp": 1695168000000
}
}
],
"link": [
{
"URL": "https://www.tandfonline.com/doi/pdf/10.1080/23744235.2023.2254380",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "301",
"original-title": [],
"page": "857-873",
"prefix": "10.1080",
"published": {
"date-parts": [
[
2023,
9,
20
]
]
},
"published-online": {
"date-parts": [
[
2023,
9,
20
]
]
},
"published-print": {
"date-parts": [
[
2023,
12,
2
]
]
},
"publisher": "Informa UK Limited",
"reference": [
{
"key": "e_1_3_4_2_1",
"unstructured": "World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard [Internet]. [cited 2023 January 16]. Available from: https://covid19.who.int/."
},
{
"key": "e_1_3_4_3_1",
"unstructured": "Ministry of Health Labour and Welfare. Situation report. [Internet]. [cited 2022 November 7]. Available from: https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou_00006.html."
},
{
"key": "e_1_3_4_4_1",
"unstructured": "Clinical management of patients with COVID-19: a guide for front-line healthcare workers. Version 2.1. JAPAN COVID-19 Case Management Guide Review Committee. [Internet]. [cited 2022 July 9]. Available from: https://www.niph.go.jp/h-crisis/wp-content/uploads/2020/07/20200706103735_content_000646510.pdf. [In Japanese]."
},
{
"DOI": "10.1038/s41586-020-03148-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_5_1"
},
{
"DOI": "10.3389/fimmu.2021.589095",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_6_1"
},
{
"DOI": "10.1016/j.mehy.2020.110412",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_7_1"
},
{
"DOI": "10.1164/rccm.202008-3265OC",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_8_1"
},
{
"DOI": "10.3390/jpm11060556",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_9_1"
},
{
"DOI": "10.1016/j.cmi.2020.06.020",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_10_1"
},
{
"DOI": "10.1515/med-2021-0250",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_11_1"
},
{
"DOI": "10.3390/jcm10214847",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_12_1"
},
{
"DOI": "10.1371/journal.pone.0249481",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_13_1"
},
{
"DOI": "10.1186/s12931-017-0708-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_14_1"
},
{
"DOI": "10.1128/JVI.01689-12",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_15_1"
},
{
"DOI": "10.12688/f1000research.23729.1",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_16_1"
},
{
"DOI": "10.1016/j.xcrm.2022.100833",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_17_1"
},
{
"key": "e_1_3_4_18_1",
"unstructured": "COVID-19 Treatment Guidelines: Corticosteroids. National Institutes of Health [Internet]. [cited 2023 July 27] Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/."
},
{
"DOI": "10.1007/s42399-020-00548-x",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_19_1"
},
{
"DOI": "10.1093/cid/ciaa829",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_20_1"
},
{
"DOI": "10.1016/S0140-6736(23)00510-X",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_21_1"
},
{
"DOI": "10.1097/RLI.0000000000000672",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_22_1"
},
{
"DOI": "10.1007/s11547-020-01320-w",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_23_1"
},
{
"DOI": "10.1016/j.acra.2021.05.014",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_24_1"
},
{
"DOI": "10.1159/000528038",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_25_1"
},
{
"DOI": "10.4049/jimmunol.0903873",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_26_1"
},
{
"DOI": "10.1172/JCI62139",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_27_1"
},
{
"DOI": "10.1038/s41586-021-03631-y",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_28_1"
},
{
"DOI": "10.1186/s40348-016-0055-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_29_1"
},
{
"DOI": "10.33611/trs.2021-018",
"doi-asserted-by": "publisher",
"key": "e_1_3_4_30_1"
}
],
"reference-count": 29,
"references-count": 29,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.tandfonline.com/doi/full/10.1080/23744235.2023.2254380"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Short-term inhalation of sargramostim with concomitant high-dose steroids does not hasten recovery in moderate COVID-19 pneumonia: a double-blind, randomised, placebo-controlled trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1080/tandf_crossmark_01",
"volume": "55"
}