Remdesivir therapy for severe pediatric COVID‐19 in Singapore: A single‐center retrospective observational cohort study
et al., Health Science Reports, doi:10.1002/hsr2.1698, Dec 2023
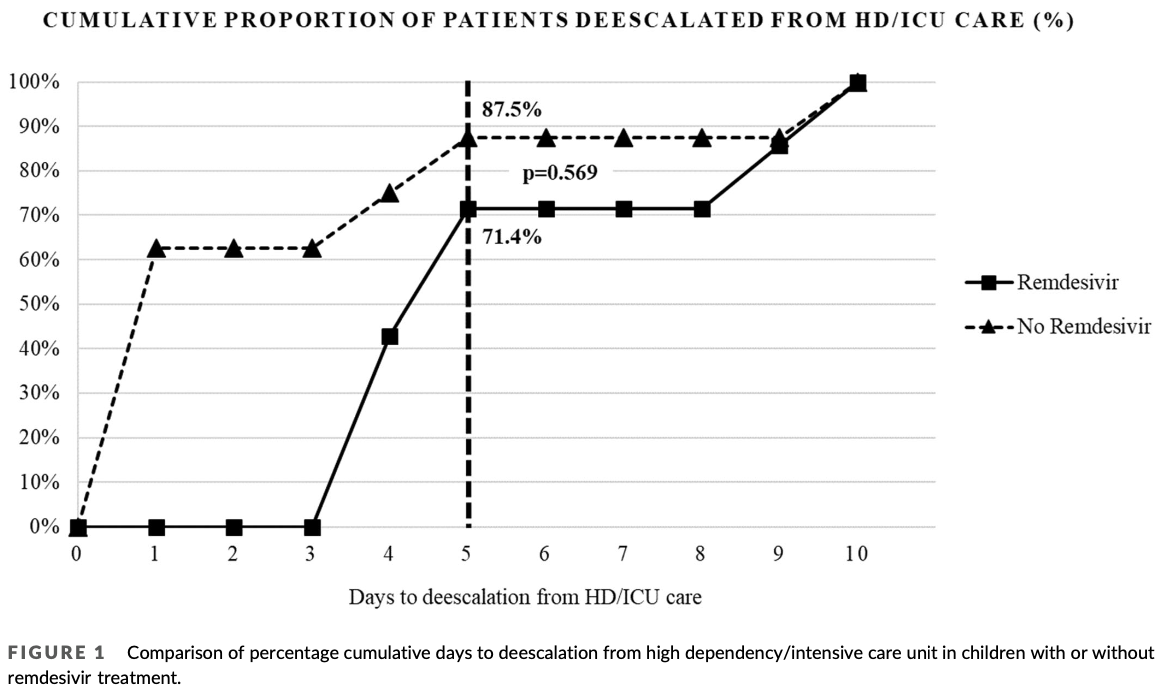
Retrospective 15 pediatric patients hospitalized for severe COVID-19 requiring oxygen and high dependency/intensive care unit (HD/ICU) admission in Singapore, showing no improvement in deescalation from HD/ICU care with remdesivir, however the remdesivir group had higher disease severity.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with significant baseline differences.
|
no deescalation, 128.6% higher, RR 2.29, p = 0.57, treatment 2 of 7 (28.6%), control 1 of 8 (12.5%), day 5.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Seah et al., 14 Dec 2023, retrospective, Singapore, peer-reviewed, median age 2.5, 9 authors, study period 1 January, 2020 - 18 March, 2022.
Contact: valerie.seah.xf@kkh.com.sg, ne.feux@gmail.com, yung.chee.fu@singhealth.com.sg.
Remdesivir therapy for severe pediatric COVID‐19 in Singapore: A single‐center retrospective observational cohort study
Health Science Reports, doi:10.1002/hsr2.1698
Background and Aims: There is a paucity of information on remdesivir (RDV) use in severe pediatric coronavirus disease 2019 (COVID-19). We aimed to explore the effectiveness of RDV as the cumulative proportion of pediatric COVID-19 patients deescalated from Day 5 of high dependency or intensive care unit (HD/ICU). Methods: All children ≤18 years admitted to Singapore's largest pediatric hospital from January 1, 2020 to March 18, 2022 were reviewed retrospectively. Patients were included if they were positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on reverse transcriptase polymerase chain reaction, required oxygen, and HD/ICU care. The characteristics and outcomes of those who received RDV or not (no-RDV) were compared. Results: We reviewed 15 children with a median age of 2.5 years (interquartile range [IQR]: 0.8-11.0), of which 7 (46.7%) received RDV. There was no difference in cumulative proportion of children deescalated from Day 5 of HD/ICU care in the RDV versus the no-RDV group (5/7, 70% vs. 7/8, 87.5%, p = 0.57). The RDV versus no-RDV group had higher disease severity, that is, WHO Ordinal Scale scores (median 6, IQR: 5-7 vs. 5, IQR: 4-5, p = 0.03), higher procalcitonin levels (ug/L) (median 4.31, IQR: 0.8-24.2 vs. 0.12, IQR: 0.09-0.26, p = 0.02), and longer HD/ ICU care days (median 5, IQR: 4-9, vs. 1, IQR: 1-4, p = 0.01). There was no significant difference in hospitalization days. There were no adverse events directly attributable to RDV. None died from COVID-19 infection.
Conclusion: Our observational analysis was unable to detect any clear benefit of RDV in terms of reducing duration in HD/ICU. RDV was well-tolerated in children with severe COVID-19.
AUTHOR CONTRIBUTIONS Valerie Xue Fen Seah: Formal analysis; methodology; supervision; writing-original draft; writing-review and editing. Rina Yue Ling Ong: Formal analysis; writing-review and editing. Kai Qian Kam: Conceptualization; methodology; supervision; writing-review and editing. Koh Cheng Thoon: Writing-review and editing. Natalie Woon Hui Tan: Writing-review and editing. Jiahui Li: Writingreview and editing. Karen Donceras Nadua: Writing-review and editing. Chia Yin Chong: Conceptualization; methodology; writingreview and editing. Chee Fu Yung: Conceptualization; formal analysis; methodology; writing-review and editing.
CONFLICT OF INTEREST STATEMENT The authors declare no conflict of interest.
References
Beigel, Tomashek, Dodd, Remdesivir for the treatment of Covid-19-final report, N Engl J Med
Bolia, Goel, Badkur, Jain, Gastrointestinal manifestations of pediatric coronavirus disease and their relationship with a severe clinical course: a systematic review and metaanalysis, J Trop Pediatr
Chow, Maust, Kazmier, Stokes, Sinus bradycardia in a pediatric patient treated with remdesivir for acute coronavirus disease 2019: a case report and a review of the literature, J Pediatric Infect Dis Soc
Goldman, Aldrich, Hagmann, Compassionate use of remdesivir in children with severe COVID-19, Pediatrics
Goldman, Lye, Hui, Remdesivir for 5 or 10 days in patients with severe COVID-19, N Engl J Med
Hegazy, Tharwat, Hassan, Clinical study to compare the efficacy and safety of casirivimab & imdevimab, remdesivir, and favipravir in hospitalized COVID-19 patients, J Clin Virol Plus
Irfan, Muttalib, Tang, Jiang, Lassi et al., Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis, Arch Dis Child
Manabe, Mizuno, Jinda, Kasai, Safety of remdesivir in 20 children with COVID-19-Case series, Biol Pharm Bull
Méndez-Echevarría, Pérez-Martínez, Del Valle, Compassionate use of remdesivir in children with COVID-19, Eur J Pediatr
Samuel, Hacker, Zebracki, Remdesivir use in pediatric patients for SARS-CoV-2 treatment: single academic center study, Pediatr Infect Dis J
Schuster, Halasa, Nakamura, A description of COVID-19-directed therapy in children admitted to US intensive care units 2020, J Pediatric Infect Dis Soc
Shekerdemian, Mahmood, Wolfe, Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units, JAMA Pediatr
Tsankov, Allaire, Irvine, Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis, Int J Infect Dis
Vangeel, Chiu, Jonghe, Remdesivir therapy for severe pediatric COVID-19 in Singapore: a single-center retrospective observational cohort study, Health Sci Rep
Vegivinti, Evanson, Lyons, Efficacy of antiviral therapies for COVID-19: a systematic review of randomized controlled trials, BMC Infect Dis
Wardell, Campbell, Vanderpluym, Dixit, Higher severe acute respiratory syndrome coronavirus 2 infection rate in pregnant patients, J Pediatric Infect Dis Soc
Zhang, Wen, Yin, Efficacy of COVID-19 treatments: a Bayesian network meta-analysis of randomized controlled trials, Front Public Health
DOI record:
{
"DOI": "10.1002/hsr2.1698",
"ISSN": [
"2398-8835",
"2398-8835"
],
"URL": "http://dx.doi.org/10.1002/hsr2.1698",
"abstract": "<jats:title>Abstract</jats:title><jats:sec><jats:title>Background and Aims</jats:title><jats:p>There is a paucity of information on remdesivir (RDV) use in severe pediatric coronavirus disease 2019 (COVID‐19). We aimed to explore the effectiveness of RDV as the cumulative proportion of pediatric COVID‐19 patients deescalated from Day 5 of high dependency or intensive care unit (HD/ICU).</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>All children ≤18 years admitted to Singapore's largest pediatric hospital from January 1, 2020 to March 18, 2022 were reviewed retrospectively. Patients were included if they were positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) on reverse transcriptase polymerase chain reaction, required oxygen, and HD/ICU care. The characteristics and outcomes of those who received RDV or not (no‐RDV) were compared.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>We reviewed 15 children with a median age of 2.5 years (interquartile range [IQR]: 0.8–11.0), of which 7 (46.7%) received RDV. There was no difference in cumulative proportion of children deescalated from Day 5 of HD/ICU care in the RDV versus the no‐RDV group (5/7, 70% vs. 7/8, 87.5%, <jats:italic>p</jats:italic> = 0.57). The RDV versus no‐RDV group had higher disease severity, that is, WHO Ordinal Scale scores (median 6, IQR: 5–7 vs. 5, IQR: 4–5, <jats:italic>p</jats:italic> = 0.03), higher procalcitonin levels (ug/L) (median 4.31, IQR: 0.8–24.2 vs. 0.12, IQR: 0.09–0.26, <jats:italic>p</jats:italic> = 0.02), and longer HD/ICU care days (median 5, IQR: 4–9, vs. 1, IQR: 1–4, <jats:italic>p</jats:italic> = 0.01). There was no significant difference in hospitalization days. There were no adverse events directly attributable to RDV. None died from COVID‐19 infection.</jats:p></jats:sec><jats:sec><jats:title>Conclusion</jats:title><jats:p>Our observational analysis was unable to detect any clear benefit of RDV in terms of reducing duration in HD/ICU. RDV was well‐tolerated in children with severe COVID‐19.</jats:p></jats:sec>",
"alternative-id": [
"10.1002/hsr2.1698"
],
"assertion": [
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Received",
"name": "received",
"order": 0,
"value": "2023-08-17"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Accepted",
"name": "accepted",
"order": 1,
"value": "2023-10-30"
},
{
"group": {
"label": "Publication History",
"name": "publication_history"
},
"label": "Published",
"name": "published",
"order": 2,
"value": "2023-12-14"
}
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-7775-6987",
"affiliation": [
{
"name": "Department of Pharmacy KK Women's and Children's Hospital Singapore Singapore"
}
],
"authenticated-orcid": false,
"family": "Seah",
"given": "Valerie Xue Fen",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Pharmacy KK Women's and Children's Hospital Singapore Singapore"
}
],
"family": "Ong",
"given": "Rina Yue Ling",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Service, Department of Paediatrics KK Women's and Children's Hospital Singapore Singapore"
},
{
"name": "Yong Loo Lin School of Medicine National University of Singapore Singapore Singapore"
},
{
"name": "Duke‐National University of Singapore Medical School Singapore Singapore"
},
{
"name": "Lee Kong Chian School of Medicine Nanyang Technological University Singapore Singapore"
}
],
"family": "Kam",
"given": "Kai Qian",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Service, Department of Paediatrics KK Women's and Children's Hospital Singapore Singapore"
},
{
"name": "Yong Loo Lin School of Medicine National University of Singapore Singapore Singapore"
},
{
"name": "Duke‐National University of Singapore Medical School Singapore Singapore"
},
{
"name": "Lee Kong Chian School of Medicine Nanyang Technological University Singapore Singapore"
}
],
"family": "Thoon",
"given": "Koh Cheng",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Service, Department of Paediatrics KK Women's and Children's Hospital Singapore Singapore"
},
{
"name": "Yong Loo Lin School of Medicine National University of Singapore Singapore Singapore"
},
{
"name": "Duke‐National University of Singapore Medical School Singapore Singapore"
},
{
"name": "Lee Kong Chian School of Medicine Nanyang Technological University Singapore Singapore"
}
],
"family": "Tan",
"given": "Natalie Woon Hui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Service, Department of Paediatrics KK Women's and Children's Hospital Singapore Singapore"
},
{
"name": "Yong Loo Lin School of Medicine National University of Singapore Singapore Singapore"
},
{
"name": "Duke‐National University of Singapore Medical School Singapore Singapore"
},
{
"name": "Lee Kong Chian School of Medicine Nanyang Technological University Singapore Singapore"
}
],
"family": "Li",
"given": "Jiahui",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Disease Service, Department of Paediatrics KK Women's and Children's Hospital Singapore Singapore"
},
{
"name": "Yong Loo Lin School of Medicine National University of Singapore Singapore Singapore"
},
{
"name": "Duke‐National University of Singapore Medical School Singapore Singapore"
},
{
"name": "Lee Kong Chian School of Medicine Nanyang Technological University Singapore Singapore"
}
],
"family": "Nadua",
"given": "Karen Donceras",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3919-3324",
"affiliation": [
{
"name": "Infectious Disease Service, Department of Paediatrics KK Women's and Children's Hospital Singapore Singapore"
},
{
"name": "Yong Loo Lin School of Medicine National University of Singapore Singapore Singapore"
},
{
"name": "Duke‐National University of Singapore Medical School Singapore Singapore"
},
{
"name": "Lee Kong Chian School of Medicine Nanyang Technological University Singapore Singapore"
}
],
"authenticated-orcid": false,
"family": "Chong",
"given": "Chia Yin",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9605-7690",
"affiliation": [
{
"name": "Infectious Disease Service, Department of Paediatrics KK Women's and Children's Hospital Singapore Singapore"
},
{
"name": "Duke‐National University of Singapore Medical School Singapore Singapore"
},
{
"name": "Lee Kong Chian School of Medicine Nanyang Technological University Singapore Singapore"
}
],
"authenticated-orcid": false,
"family": "Yung",
"given": "Chee Fu",
"sequence": "additional"
}
],
"container-title": "Health Science Reports",
"container-title-short": "Health Science Reports",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"onlinelibrary.wiley.com"
]
},
"created": {
"date-parts": [
[
2023,
12,
14
]
],
"date-time": "2023-12-14T11:34:31Z",
"timestamp": 1702553671000
},
"deposited": {
"date-parts": [
[
2023,
12,
14
]
],
"date-time": "2023-12-14T11:34:47Z",
"timestamp": 1702553687000
},
"indexed": {
"date-parts": [
[
2023,
12,
15
]
],
"date-time": "2023-12-15T00:40:48Z",
"timestamp": 1702600848612
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2023,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2023,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 13,
"start": {
"date-parts": [
[
2023,
12,
14
]
],
"date-time": "2023-12-14T00:00:00Z",
"timestamp": 1702512000000
}
}
],
"link": [
{
"URL": "https://onlinelibrary.wiley.com/doi/pdf/10.1002/hsr2.1698",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"prefix": "10.1002",
"published": {
"date-parts": [
[
2023,
12
]
]
},
"published-online": {
"date-parts": [
[
2023,
12,
14
]
]
},
"published-print": {
"date-parts": [
[
2023,
12
]
]
},
"publisher": "Wiley",
"reference": [
{
"key": "e_1_2_11_2_1",
"unstructured": "World Health Organization. Coronavirus disease 2019 (COVID‐19) situation report–51. World Health Organization. March 11 2020. Accessed 10 May 2022. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf"
},
{
"key": "e_1_2_11_3_1",
"unstructured": "American Academy of Pediatrics. Children and COVID‐19: State‐Level Data Report. 5 May 2022. Accessed 10 May 2022. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/"
},
{
"DOI": "10.1136/archdischild-2020-321385",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_4_1"
},
{
"DOI": "10.1056/NEJMoa2007764",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_5_1"
},
{
"DOI": "10.1056/NEJMoa2015301",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_6_1"
},
{
"key": "e_1_2_11_7_1",
"unstructured": "Study to evaluate the safety tolerability pharmacokinetics and efficacy of remdesivir (GS‐5734™) in participants from birth to <18 years of age with coronavirus disease 2019 (COVID‐19) (CARAVAN). Accessed 10 May 2022.https://www.clinicaltrials.gov/ct2/show/NCT04431453"
},
{
"DOI": "10.1542/peds.2020-047803",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_8_1"
},
{
"DOI": "10.1007/s00431-020-03876-1",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_9_1"
},
{
"DOI": "10.1248/bpb.b22-00470",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_10_1"
},
{
"DOI": "10.1097/INF.0000000000003814",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_11_1"
},
{
"DOI": "10.1002/cpu.30542",
"doi-asserted-by": "crossref",
"key": "e_1_2_11_12_1",
"unstructured": "US Food and Drug Administration. FDA approves first treatment for COVID‐19. October 22 2020. Accessed May 10 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19"
},
{
"key": "e_1_2_11_13_1",
"unstructured": "Fact sheet for healthcare providers emergency use authorization (EUA) of Veklury® (remdesivir) for hospitalized pediatric patients weighing 3.5 kg to less than 40 kg or hospitalized pediatric patients less than 12 years of age weighing at least 3.5 kg. 2020. Accessed May 10 2022.https://www.fda.gov/media/137566/download"
},
{
"key": "e_1_2_11_14_1",
"unstructured": "Health Sciences Authority Singapore. Conditional approval of remdesivir (Veklury®) for COVID‐19 infection in Singapore. September 17 2020. Accessed May 10 2022.https://www.hsa.gov.sg/announcements/safety-alert/conditional-approval-of-remdesivir-(veklury-)-for-covid-19-infection-in-singapore"
},
{
"key": "e_1_2_11_15_1",
"unstructured": "COVID‐19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID‐19). Clinical spectrum of SARS‐CoV‐2 infection. National Institutes of Health. Accessed May 10 2022.https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/"
},
{
"key": "e_1_2_11_16_1",
"unstructured": "World Health Organization. WHO R&D blueprint novel coronavirus COVID‐19 therapeutic trial synopsis 2020. Accessed May 10 2022.https://cdn.who.int/media/docs/default-source/blue-print/covid-19-therapeutic-trial-synopsis.pdf"
},
{
"key": "e_1_2_11_17_1",
"unstructured": "COVID‐19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID‐19) treatment guidelines. National Institutes of Health. Accessed May 10 2022.https://www.covid19treatmentguidelines.nih.gov/"
},
{
"DOI": "10.1093/jpids/piaa084",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_18_1"
},
{
"DOI": "10.1016/j.ijid.2020.11.163",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_19_1"
},
{
"DOI": "10.1001/jamapediatrics.2020.1948",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_20_1"
},
{
"DOI": "10.1016/j.jcvp.2023.100151",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_21_1"
},
{
"DOI": "10.1093/jpids/piab123",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_22_1"
},
{
"DOI": "10.3389/fpubh.2021.729559",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_23_1"
},
{
"DOI": "10.1186/s12879-022-07068-0",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_24_1"
},
{
"DOI": "10.1093/tropej/fmab051",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_25_1"
},
{
"DOI": "10.1093/jpids/piab029",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_26_1"
},
{
"key": "e_1_2_11_27_1",
"unstructured": "Ministry of Health Singapore. COVID‐19 epidemic curve. Accessed June 16 2022.https://www.moh.gov.sg/covid-19/statistics"
},
{
"DOI": "10.1016/j.antiviral.2022.105252",
"doi-asserted-by": "publisher",
"key": "e_1_2_11_28_1"
}
],
"reference-count": 27,
"references-count": 27,
"relation": {},
"resource": {
"primary": {
"URL": "https://onlinelibrary.wiley.com/doi/10.1002/hsr2.1698"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"General Medicine"
],
"subtitle": [],
"title": "Remdesivir therapy for severe pediatric COVID‐19 in Singapore: A single‐center retrospective observational cohort study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1002/crossmark_policy",
"volume": "6"
}