Influence of conflicts of interest on public positions in the COVID-19 era, the case of Gilead Sciences
et al., New Microbes and New Infections, Volume 38, doi:10.1016/j.nmni.2020.100710
, Jun 2020
HCQ for COVID-19
1st treatment shown to reduce risk in
March 2020, now with p < 0.00000000001 from 424 studies, used in 59 countries.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
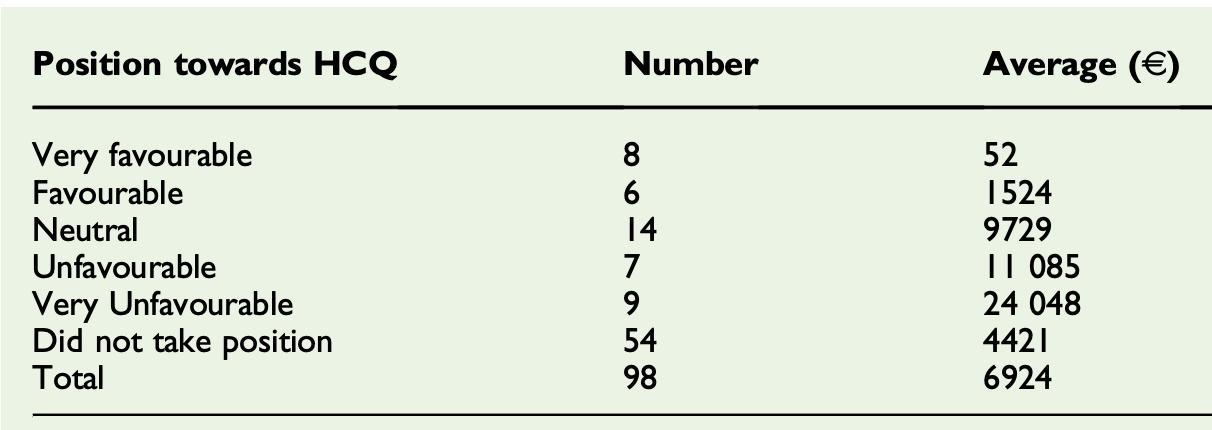
Shows a correlation (Spearman test, p = 0.017) between the amount received from Gilead Sciences and public opposition to the use of HCQ in France.
1.
Mothae et al., SARS-CoV-2 host-pathogen interactome: insights into more players during pathogenesis, Virology, doi:10.1016/j.virol.2025.110607.
2.
Monsalve et al., NETosis: A key player in autoimmunity, COVID-19, and long COVID, Journal of Translational Autoimmunity, doi:10.1016/j.jtauto.2025.100280.
3.
Xie et al., The role of reactive oxygen species in severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) infection-induced cell death, Cellular & Molecular Biology Letters, doi:10.1186/s11658-024-00659-6.
4.
Gkioulekas et al., Use of hydroxychloroquine in multidrug protocols for SARS-CoV-2, Tasman Medical Journal, 6:4, tasmanmedicaljournal.com/2024/10/use-of-hydroxychloroquine-in-multidrug-protocols-for-sars-cov-2a/.
5.
Gortler et al., Those Published “17,000 Hydroxychloroquine Deaths” Never Happened, Brownstone Journal, brownstone.org/articles/those-published-17000-hydroxychloroquine-deaths-never-happened/.
6.
Boretti et al., Correct Use of HCQ Did Not Cause Extra Fatalities in COVID-19 Infection, Coronaviruses, doi:10.2174/0126667975327612240902104505.
7.
Gortler (B) et al., Trump’s 63 Million Doses of Hydroxychloroquine Could Have Been Great for America, Brownstone Journal, brownstone.org/articles/trumps-63-million-doses-of-hydroxychloroquine-could-have-been-great-for-america/.
8.
Enyeji et al., Effective Treatment of COVID-19 Infection with Repurposed Drugs: Case Reports, Viral Immunology, doi:10.1089/vim.2024.0034.
9.
Asaba et al., Interplay of TLR4 and SARS-CoV-2: Unveiling the Complex Mechanisms of Inflammation and Severity in COVID-19 Infections, Journal of Inflammation Research, doi:10.2147/jir.s474707.
10.
Scheim et al., Back to the Basics of SARS-CoV-2 Biochemistry: Microvascular Occlusive Glycan Bindings Govern Its Morbidities and Inform Therapeutic Responses, Viruses, doi:10.3390/v16040647.
11.
Ali et al., SARS-CoV-2 Syncytium under the Radar: Molecular Insights of the Spike-Induced Syncytia and Potential Strategies to Limit SARS-CoV-2 Replication, Journal of Clinical Medicine, doi:10.3390/jcm12186079.
12.
Brouqui et al., There is no such thing as a Ministry of Truth and why it is important to challenge conventional “wisdom” - A personal view, New Microbes and New Infections, doi:10.1016/j.nmni.2023.101155.
13.
Loo et al., Recent Advances in Inhaled Nanoformulations of Vaccines and Therapeutics Targeting Respiratory Viral Infections, Pharmaceutical Research, doi:10.1007/s11095-023-03520-1.
14.
Boretti (B), A., Pharmacotherapy for Covid-19 infection in the countries of the Cooperation Council for the Arab States, Journal of Taibah University Medical Sciences, doi:10.1016/j.jtumed.2021.08.005.
15.
Vigbedor et al., Review of four major biomolecular target sites for COVID-19 and possible inhibitors as treatment interventions, Journal of Applied Pharmaceutical Science, doi:10.7324/JAPS.2021.110825.
16.
Kaur et al., Folic acid as placebo in controlled clinical trials of hydroxychloroquine prophylaxis in COVID-19: Is it scientifically justifiable?, Medical Hypotheses, doi:10.1016/j.mehy.2021.110539.
17.
Raoult, D., Rational for meta-analysis and randomized treatment: the COVID-19 example, Clinical Microbiology and Infection, doi:10.1016/j.cmi.2020.10.012.
18.
Matada et al., A comprehensive review on the biological interest of quinoline and its derivatives, Bioorganic & Medicinal Chemistry, doi:10.1016/j.bmc.2020.115973.
19.
IHU, Natural history and therapeutic options for COVID-19, Expert Review of Clinical Immunology, www.mediterranee-infection.com/wp-content/uploads/2020/09/ERM-2020-0073.R1_Proof_hi.pdf.
20.
Hecel et al., Zinc(II)—The Overlooked Éminence Grise of Chloroquine’s Fight against COVID-19?, Pharmaceuticals, 13:9, 228, doi:10.3390/ph13090228.
21.
Li et al., Is hydroxychloroquine beneficial for COVID-19 patients?, Cell Death & Disease volume 11, doi:10.1038/s41419-020-2721-8.
22.
Goldstein, L., Hydroxychloroquine-based COVID-19 Treatment, A Systematic Review of Clinical Evidence and Expert Opinion from Physicians’ Surveys, Preprint, July 7, 2020, wattsupwiththat.com/2020/07/07/hydroxychloroquine-based-covid-19-treatment-a-systematic-review-of-clinical-evidence-and-expert-opinion-from-physicians-surveys/.
23.
Roussel et al., Influence of conflicts of interest on public positions in the COVID-19 era, the case of Gilead Sciences, New Microbes and New Infections, Volume 38
, doi:10.1016/j.nmni.2020.100710.
24.
Mo et al., Chloroquine phosphate: therapeutic drug for COVID-19, Journal of Southern Medical University, doi:10.12122/j.issn.1673-4254.2020.04.22.
25.
Gao et al., Update on Use of Chloroquine/Hydroxychloroquine to Treat Coronavirus Disease 2019 (COVID-19), Biosci Trends, May 21, 2020, 14:2, 156-158, doi:10.5582/bst.2020.03072.
26.
Derwand et al., Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today's battle against COVID-19?, Medical Hypotheses, doi:10.1016/j.mehy.2020.109815.
27.
Sahraei et al., Aminoquinolines against coronavirus disease 2019 (COVID-19): chloroquine or hydroxychloroquine, International Journal of Antimicrobial Agents, April 2020, 55:4, doi:10.1016/j.ijantimicag.2020.105945.
28.
Todaro et al., An Effective Treatment for Coronavirus (COVID-19), 3/13, github.com/covidtrial/info/raw/master/An%20Effective%20Treatment%20for%20Coronavirus%20(COVID-19).pdf.
Roussel et al., 6 Jun 2020, peer-reviewed, 2 authors.
Influence of conflicts of interest on public positions in the COVID-19 era, the case of Gilead Sciences
New Microbes and New Infections, doi:10.1016/j.nmni.2020.100710
Funding and gifts from the pharmaceutical industry have an influence on the decisions made by physicians and medical experts. In the context of the coronavirus disease 2019 epidemic, several treatments are available to treat patients infected with the virus. Some are protected by patents, such as remdesivir, others0020stare not, such as hydroxychloroquine. We wanted to observe the possible correlation between the fact, for an academic doctor in infectious diseases, of having benefited from funding by Gilead Sciences, producer of remdesivir, and the public positions taken by this doctor towards hydroxychloroquine. Our results show a correlation (Spearman test, p = 0.017) between the amount received from the Gilead Sciences company and public opposition to the use of hydroxychloroquine in France. This should open up the debate on the role of the interest links of doctors with pharmaceutical companies in the medical and scientific public debate.
Conflicts of interest There is no conflict of interest.
References
Blumenthal, Doctors and drug companies, N Engl J Med
Cao, Wang, Wen, Liu, Wang et al., A Trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19
Chen, Hu, Zhang, Jiang, Han et al., Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, medRxiv, doi:10.1101/2020.03.22.20040758
Chen, Liu, Lui, Liu, Xu et al., A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19), J Zhejiang Univ (Med Sci, doi:10.3785/j.issn.1008-9292.2020.03.03118
Dana, Loewenstein, A social science perspective on gifts to physicians from industry, JAMA
De Novales, Ramírez-Olivencia, Estébanez, De Dios, Herrero et al., Early hydroxychloroquine is associated with an increase of survival in COVID-19 patients: an observational study, Preprints, doi:10.20944/preprints202005.0057.v1
Dejong, Aguilar, Tseng, Lin, Boscardin et al., Pharmaceutical industry-sponsored meals and physician prescribing patterns for medicare beneficiaries [published correction appears in, JAMA Intern Med
Edwards, Remdesivir shows promising results for coronavirus
Friedman, There's No such thing as a free lunch
Gautret, Lagier, Parola, Hoang, Meddeb et al., Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study, Travel Med Infect Dis
Gautret, Lagier, Parola, Hoang, Meddeb et al., Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial, Int J Antimicrob Agents
Gibbons, Landry, Blouch, Jones, Williams et al., A comparison of physicians' and patients' attitudes toward pharmaceutical industry gifts, J Gen Intern Med
Huang, Li, Xiao, Liang, Pang et al., None, doi:10.1101/2020.04.26.20081059v1
Huang, Tang, Pang, Li, Ma et al., Treating COVID-19 with chloroquine, J Mol Cell Biol, doi:10.1093/jmcb/mjaa014
Katz, Caplan, Merz, All gifts large and small: toward an understanding of the ethics of pharmaceutical industry gift-giving, Am J Bioeth
Lexchin, Interactions between physicians and the pharmaceutical industry: what does the literature say?, CMAJ
Magagnoli, Narendran, Pereira, Cummings, Hardin et al., Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, medRxiv, doi:10.1101/2020.04.16.20065920
Mahevas, Tran, Roumier, Chabrol, Paule et al., No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial, medRxiv, doi:10.1101/2020.04.10.20060699
Million, Lagier, Gautret, Colson, Fournier et al., Full-length title: early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France, Travel Med Infect Dis, doi:10.1016/j.tmaid.2020.101738
Sharma, Vadhariya, Johnson, Marcum, Holmes, Association between industry payments and prescribing costly medications: an observational study using open payments and medicare part D data, BMC Health Serv Res
Steinman, Shlipak, Mcphee, Of principles and pens: attitudes and practices of medicine housestaff toward pharmaceutical industry promotions, Am J Med
Tang, Cao, Han, Wang, Chen et al., Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial, medRxiv, doi:10.1101/2020.04.10.20060558
Wang, Cao, Zhang, Yang, Liu et al., Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro, Cell Res
Wang, Zhang, Du, Du, Zhao et al., Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebocontrolled, multicentre trial, The Lancet, doi:10.1016/S0140-6736(20)31022-9
Watkins, Characteristics of general practitioners who frequently see drug industry representatives: national cross sectional study, BMJ
Yu, Wang, Li, Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19, doi:10.1101/2020.04.27.20073379v1
DOI record:
{
"DOI": "10.1016/j.nmni.2020.100710",
"ISSN": [
"2052-2975"
],
"URL": "http://dx.doi.org/10.1016/j.nmni.2020.100710",
"alternative-id": [
"S2052297520300627"
],
"article-number": "100710",
"author": [
{
"affiliation": [],
"family": "Roussel",
"given": "Y.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Raoult",
"given": "D.",
"sequence": "additional"
}
],
"container-title": "New Microbes and New Infections",
"container-title-short": "New Microbes and New Infections",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2020,
6,
6
]
],
"date-time": "2020-06-06T15:20:38Z",
"timestamp": 1591456838000
},
"deposited": {
"date-parts": [
[
2020,
12,
25
]
],
"date-time": "2020-12-25T23:32:42Z",
"timestamp": 1608939162000
},
"funder": [
{
"DOI": "10.13039/501100001665",
"doi-asserted-by": "publisher",
"name": "National Agency for Research"
}
],
"indexed": {
"date-parts": [
[
2024,
2,
1
]
],
"date-time": "2024-02-01T17:40:54Z",
"timestamp": 1706809254688
},
"is-referenced-by-count": 13,
"issued": {
"date-parts": [
[
2020,
11
]
]
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
11,
1
]
],
"date-time": "2020-11-01T00:00:00Z",
"timestamp": 1604188800000
}
},
{
"URL": "http://creativecommons.org/licenses/by-nc-nd/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2020,
6,
5
]
],
"date-time": "2020-06-05T00:00:00Z",
"timestamp": 1591315200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2052297520300627?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2052297520300627?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "100710",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2020,
11
]
]
},
"published-print": {
"date-parts": [
[
2020,
11
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Interactions between physicians and the pharmaceutical industry: what does the literature say?",
"author": "Lexchin",
"first-page": "1401",
"journal-title": "CMAJ",
"key": "10.1016/j.nmni.2020.100710_bib1",
"volume": "149",
"year": "1993"
},
{
"DOI": "10.1001/jama.290.2.252",
"article-title": "A social science perspective on gifts to physicians from industry",
"author": "Dana",
"doi-asserted-by": "crossref",
"first-page": "252",
"journal-title": "JAMA",
"key": "10.1016/j.nmni.2020.100710_bib2",
"volume": "290",
"year": "2003"
},
{
"DOI": "10.1056/NEJMhpr042734",
"article-title": "Doctors and drug companies",
"author": "Blumenthal",
"doi-asserted-by": "crossref",
"first-page": "1885",
"journal-title": "N Engl J Med",
"key": "10.1016/j.nmni.2020.100710_bib3",
"volume": "351",
"year": "2004"
},
{
"DOI": "10.1162/15265160360706552",
"article-title": "All gifts large and small: toward an understanding of the ethics of pharmaceutical industry gift-giving",
"author": "Katz",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "Am J Bioeth",
"key": "10.1016/j.nmni.2020.100710_bib4",
"volume": "3",
"year": "2003"
},
{
"author": "Friedman",
"key": "10.1016/j.nmni.2020.100710_bib5",
"series-title": "There's No such thing as a free lunch",
"year": "1975"
},
{
"DOI": "10.1136/bmj.326.7400.1178",
"article-title": "Characteristics of general practitioners who frequently see drug industry representatives: national cross sectional study",
"author": "Watkins",
"doi-asserted-by": "crossref",
"first-page": "1178",
"issue": "7400",
"journal-title": "BMJ",
"key": "10.1016/j.nmni.2020.100710_bib6",
"volume": "326",
"year": "2003"
},
{
"DOI": "10.1186/s12913-018-3043-8",
"article-title": "Association between industry payments and prescribing costly medications: an observational study using open payments and medicare part D data",
"author": "Sharma",
"doi-asserted-by": "crossref",
"first-page": "236",
"journal-title": "BMC Health Serv Res",
"key": "10.1016/j.nmni.2020.100710_bib7",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.1001/jamainternmed.2016.2765",
"article-title": "Pharmaceutical industry-sponsored meals and physician prescribing patterns for medicare beneficiaries [published correction appears in JAMA Intern Med 2016;176:1411–12]",
"author": "DeJong",
"doi-asserted-by": "crossref",
"first-page": "1114",
"journal-title": "JAMA Intern Med",
"key": "10.1016/j.nmni.2020.100710_bib8",
"volume": "176",
"year": "2016"
},
{
"DOI": "10.1046/j.1525-1497.1998.00048.x",
"article-title": "A comparison of physicians' and patients' attitudes toward pharmaceutical industry gifts",
"author": "Gibbons",
"doi-asserted-by": "crossref",
"first-page": "151",
"journal-title": "J Gen Intern Med",
"key": "10.1016/j.nmni.2020.100710_bib9",
"volume": "13",
"year": "1998"
},
{
"DOI": "10.1016/S0002-9343(01)00660-X",
"article-title": "Of principles and pens: attitudes and practices of medicine housestaff toward pharmaceutical industry promotions",
"author": "Steinman",
"doi-asserted-by": "crossref",
"first-page": "551",
"journal-title": "Am J Med",
"key": "10.1016/j.nmni.2020.100710_bib10",
"volume": "110",
"year": "2001"
},
{
"DOI": "10.1056/NEJMoa2001282",
"article-title": "A Trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19",
"author": "Cao",
"doi-asserted-by": "crossref",
"journal-title": "N Engl J Med",
"key": "10.1016/j.nmni.2020.100710_bib12",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101738",
"article-title": "Full-length title: early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France",
"author": "Million",
"doi-asserted-by": "crossref",
"journal-title": "Travel Med Infect Dis",
"key": "10.1016/j.nmni.2020.100710_bib13",
"year": "2020"
},
{
"DOI": "10.1038/s41422-020-0282-0",
"article-title": "Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro",
"author": "Wang",
"doi-asserted-by": "crossref",
"first-page": "269",
"journal-title": "Cell Res",
"key": "10.1016/j.nmni.2020.100710_bib14",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1016/j.ijantimicag.2020.105949",
"article-title": "Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial",
"author": "Gautret",
"doi-asserted-by": "crossref",
"first-page": "105949",
"journal-title": "Int J Antimicrob Agents",
"key": "10.1016/j.nmni.2020.100710_bib15",
"year": "2020"
},
{
"DOI": "10.1016/j.tmaid.2020.101663",
"article-title": "Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study",
"author": "Gautret",
"doi-asserted-by": "crossref",
"first-page": "101663",
"journal-title": "Travel Med Infect Dis",
"key": "10.1016/j.nmni.2020.100710_bib16",
"year": "2020"
},
{
"article-title": "Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial",
"author": "Chen",
"first-page": "20040758",
"issue": "22",
"journal-title": "medRxiv",
"key": "10.1016/j.nmni.2020.100710_bib17",
"volume": "3",
"year": "2020"
},
{
"article-title": "A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19)",
"author": "Chen",
"journal-title": "J Zhejiang Univ (Med Sci",
"key": "10.1016/j.nmni.2020.100710_bib18",
"volume": "vol. 49",
"year": "2020"
},
{
"article-title": "Treating COVID-19 with chloroquine",
"author": "Huang",
"first-page": "322",
"issue": "4",
"journal-title": "J Mol Cell Biol",
"key": "10.1016/j.nmni.2020.100710_bib19",
"volume": "12",
"year": "2020"
},
{
"article-title": "Hydroxychloroquine in patients with COVID-19: an open-label, randomized, controlled trial",
"author": "Tang",
"journal-title": "medRxiv",
"key": "10.1016/j.nmni.2020.100710_bib20",
"volume": "4",
"year": "2020"
},
{
"article-title": "No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial",
"author": "Mahevas",
"journal-title": "medRxiv",
"key": "10.1016/j.nmni.2020.100710_bib21",
"volume": "4",
"year": "2020"
},
{
"article-title": "Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19",
"author": "Magagnoli",
"journal-title": "medRxiv",
"key": "10.1016/j.nmni.2020.100710_bib22",
"year": "2020"
},
{
"author": "Edwards",
"key": "10.1016/j.nmni.2020.100710_bib23",
"series-title": "Remdesivir shows promising results for coronavirus, Fauci says, NBC News",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)31022-9",
"article-title": "Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial",
"author": "Wang",
"doi-asserted-by": "crossref",
"journal-title": "The Lancet",
"key": "10.1016/j.nmni.2020.100710_bib24",
"year": "2020"
},
{
"DOI": "10.1101/2020.04.27.20073379",
"doi-asserted-by": "crossref",
"key": "10.1016/j.nmni.2020.100710_bib25",
"unstructured": "Yu B, Wang DW, Li C. Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID-19. MedrXiv Posted as a preprint 1 May 2020. https://www.medrxiv.org/content/10.1101/2020.04.27.20073379v1."
},
{
"author": "Huang",
"key": "10.1016/j.nmni.2020.100710_bib26",
"series-title": "MedrXiv",
"year": "2020"
},
{
"article-title": "Early hydroxychloroquine is associated with an increase of survival in COVID-19 patients: an observational study",
"author": "Membrillo de Novales",
"first-page": "2020050057",
"journal-title": "Preprints",
"key": "10.1016/j.nmni.2020.100710_bib27",
"year": "2020"
}
],
"reference-count": 26,
"references-count": 26,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2052297520300627"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Influence of conflicts of interest on public positions in the COVID-19 era, the case of Gilead Sciences",
"type": "journal-article",
"volume": "38"
}
