Efficient Control of IL-6, CRP and Ferritin in COVID-19 Patients With Two Variants of Beta-1,3-1,6 Glucans in Combination: An Open-Label, Prospective, Randomised Clinical Trial
et al., Global Advances in Integrative Medicine and Health, doi:10.1177/27536130251327134, Jan 2025
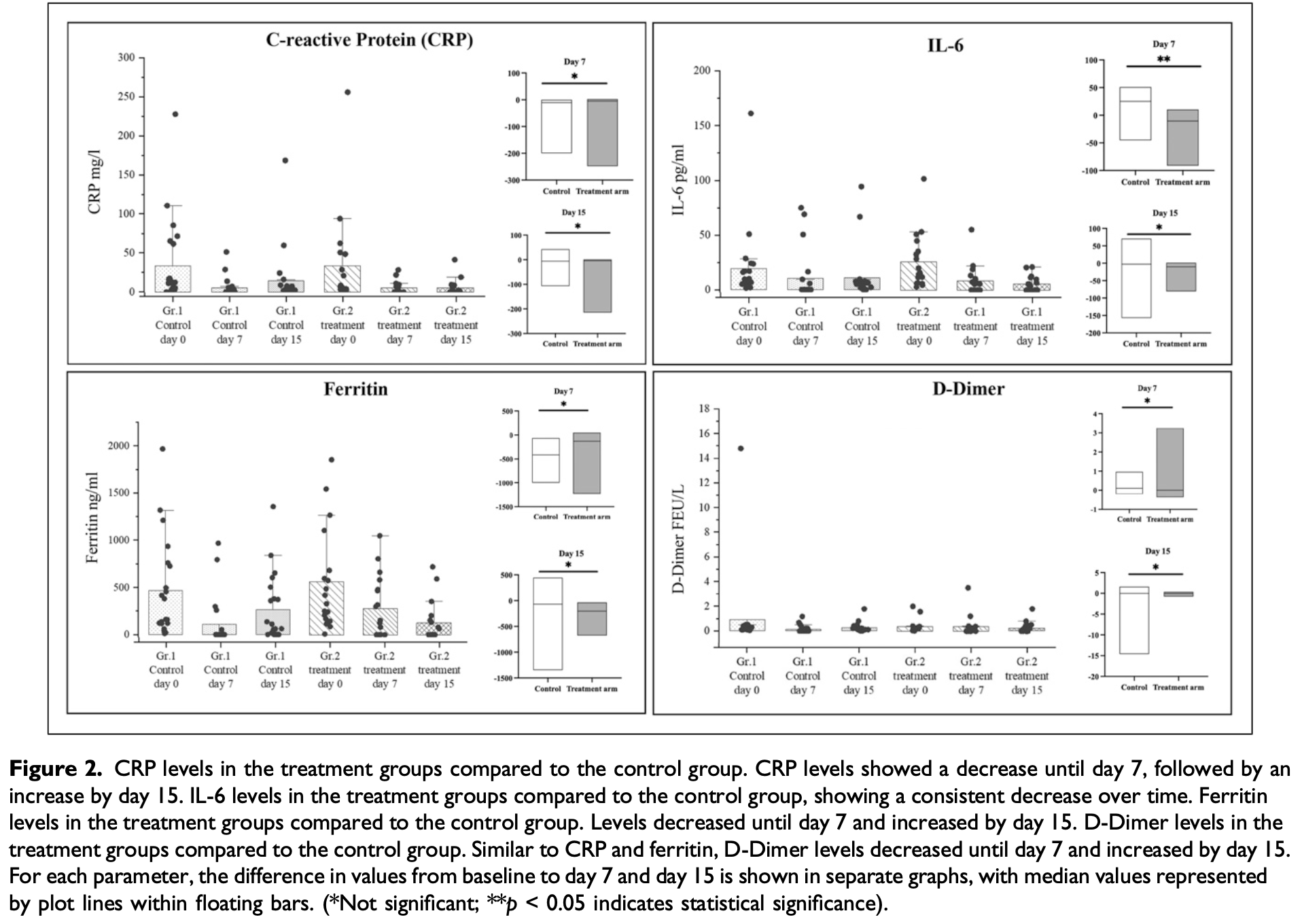
RCT 40 COVID-19 patients showing significantly decreased IL-6 levels at day 7 with the combination of AFO-202 and N-163 beta-glucans compared to standard treatment, however there were no significant differences in clinical outcomes.
|
risk of death, 222.2% higher, RR 3.22, p = 0.45, treatment 1 of 18 (5.6%), control 0 of 22 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of mechanical ventilation, 444.4% higher, RR 5.44, p = 0.20, treatment 2 of 18 (11.1%), control 0 of 22 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
risk of ICU admission, 444.4% higher, RR 5.44, p = 0.20, treatment 2 of 18 (11.1%), control 0 of 22 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pushkala et al., 31 Jan 2025, Randomized Controlled Trial, India, peer-reviewed, 10 authors, study period October 2021 - March 2022.
Contact: drsam@nichimail.jp, drspp@nichimail.jp.
Efficient Control of IL-6, CRP and Ferritin in COVID-19 Patients With Two Variants of Beta-1,3-1,6 Glucans in Combination: An Open-Label, Prospective, Randomised Clinical Trial
Global Advances in Integrative Medicine and Health, doi:10.1177/27536130251327134
Background: Several biomarkers, including C-reactive protein (CRP), ferritin, D-dimer, and Interleukin-6 (IL-6), are established predictors of disease severity and respiratory failure in patients with COVID-19. Objective: In this randomised clinical study, we evaluated the efficiency of the combination of 2 variants' AFO-202 and N-163 strains of Aureobasidium pullulans produced 1,3-1,6 β-glucans in comparison with the control arm on these biomarkers in COVID-19 patients. Methods: Forty RT-PCR positive COVID-19 patients were divided into 2 groups: control (n = 22) and standard treatment; ii. (n = 18) -Standard treatment + combination of AFO-202 and N-163 beta glucans for 15 days. Results: IL-6 levels significantly decreased in the treatment group on day 7 (P = 0.03) but not by day 15 (P = 0.30). CRP levels in the treatment group decreased at day 7 (5.53 ± 8.21 mg/L) compared to baseline but showed no significant difference from the control group (4.91 ± 12.54 mg/L, P = 0.98). At day 15, CRP levels remained lower in the treatment group (5.42 ± 10.41 mg/L) but increased in the control group (14.0 ± 37.16 mg/L), with no significant difference (P = 0.52). Ferritin levels dropped
Author Contributions S.U.P and S.A. contributed to conception and design of the study. E.T and D. S helped with data collection. S.A and S.P. drafted the manuscript. K.R, S.S, V. D, N.I and M. I performed critical revision of the manuscript. All the authors read, and approved the submitted version. All the authors meet the criteria for authorship as per the ICMJE criteria
Declaration of conflicting interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Author Samuel Abraham is a shareholder in GN Corporation, Japan which in turn is a shareholder in the manufacturing company of novel beta glucans using different strains of Aureobasidium pullulans.
Ethical Statement
Ethical Approval The study was registered in Clinical trials registry of India, CTRI/ 2021/10/037380. The study was approved by the Institutional Ethics Committee (IEC) of Madras Medical College, India on 15 th September, 2021.
ORCID iD Samuel JK Abraham https://orcid.org/0000-0003-2646-2687
Supplemental Material Supplemental material for this article is available online.
References
Chung, Thone, Kwon, COVID-19 vaccines: the status and perspectives in delivery points of view, Adv Drug Deliv Rev
Dedeepiya, Sivaraman, Venkatesh, Preethy, Abraham, Potential effects of nichi glucan as a food supplement for diabetes mellitus and hyperlipidemia: preliminary findings from the study on three patients from India, Case Rep Med, doi:10.1155/2012/895370
Elbadawy, Khattab, El-Agamy, IL-6 at the center of cytokine storm: circulating inflammation mediators as biomarkers in hospitalized COVID-19 patients, J Clin Lab Anal, doi:10.1002/jcla.24881
Ganesh, Rao, Ravikumar, Beneficial effects of Black yeast derived 1-3, 1-6 beta glucan-Nichi Glucan in a dyslipidemic individual of Indian origin -a case report, J Diet Suppl
Ikewaki, Ikeue, Nagataki, Beneficial effects of 1,3-1,6 β-glucans produced by Aureobasidium pullulans on nonesterified fatty acid levels in diabetic KKAy mice and their potential implications in metabolic dysregulation, J Diabetes Metab Disord
Ikewaki, Iwasaki, Kurosawa, β-glucans: widespectrum immune-balancing food-supplement-based enteric (β-WIFE) vaccine adjuvant approach to COVID-19, Hum Vaccines Immunother
Ikewaki, Levy, Kurosawa, Hepatoprotective effects of Aureobasidium pullulans derived β 1,3-1,6 glucans in a murine model of non-alcoholic steatohepatitis, J Clin Exp Hepatol
Ikewaki, Raghavan, Dedeepiya, Beneficial immune-regulatory effects of novel strains of Aureobasidium pullulans AFO-202 and N-163 produced beta glucans in Sprague Dawley rats, Clinical Immunology Communications, doi:10.1016/j.clicom.2021.11.001
Ikewaki, Rao, Archibold, Coagulopathy associated with COVID-19 -perspectives & Preventive strategies using a biological response modifier Glucan, Thromb J
Ikewaki, Sonoda, Kurosawa, Beta 1,3-1,6 glucans produced by two novel strains of Aureobasidium pullulans exert immune and metabolic beneficial effects in healthy middle-aged Japanese men: results of an exploratory randomized control study, JAR Life
Khoury, Cuda, Luhovyy, Anderson, Beta glucan: health benefits in obesity and metabolic syndrome, J Nutr Metab, doi:10.1155/2012/851362
Marco Castro, Calder, Roche, β-1,3/1,6-Glucans and immunity: state of the art and future directions, Mol Nutr Food Res, doi:10.1002/mnfr.201901071
Preethy, Raghavan, Dedeepiya, Beneficial immune regulation by biological response modifier glucans in COVID-19 and their envisaged potentials in the management of sepsis, Front Immunol
Raghavan, Dedeepiya, Suryaprakash, Beneficial effects of novel aureobasidium pullulans strains produced beta-1,3-1,6 glucans on interleukin-6 and D-dimer levels in COVID-19 patients; results of a randomized multiple-arm pilot clinical study, Biomed Pharmacother, doi:10.1016/j.biopha.2021.112243
Sabaka, Koščálová, Straka, Role of interleukin 6 as a predictive factor for a severe course of Covid-19: retrospective data analysis of patients from a long-term care facility during Covid-19 outbreak, BMC Infect Dis
Tang, Yin, Hu, Mei, Controlling cytokine storm is vital in COVID-19, Front Immunol
Vargas-Vargas, Cortés-Rojo, Ferritin levels and COVID-19, Rev Panam Salud Públic
Vetvicka, Fernandez-Botran, β-Glucan and parasites, Helminthologia, doi:10.2478/helm-2018-0021
Zhao, Xing, Influence of fasting plasma glucose level on admission of COVID-19 patients: a retrospective study, J Diabetes Res
DOI record:
{
"DOI": "10.1177/27536130251327134",
"ISSN": [
"2753-6130",
"2753-6130"
],
"URL": "http://dx.doi.org/10.1177/27536130251327134",
"abstract": "<jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Several biomarkers, including C-reactive protein (CRP), ferritin, D-dimer, and Interleukin-6 (IL-6), are established predictors of disease severity and respiratory failure in patients with COVID-19.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Objective</jats:title>\n <jats:p>\n In this randomised clinical study, we evaluated the efficiency of the combination of 2 variants’ AFO-202 and N-163 strains of\n <jats:italic>Aureobasidium pullulans</jats:italic>\n produced 1,3-1,6 β-glucans in comparison with the control arm on these biomarkers in COVID-19 patients.\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>Forty RT-PCR positive COVID-19 patients were divided into 2 groups: control (n = 22) and standard treatment; ii. (n = 18) – Standard treatment + combination of AFO-202 and N-163 beta glucans for 15 days.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>\n IL-6 levels significantly decreased in the treatment group on day 7 (\n <jats:italic>P</jats:italic>\n = 0.03) but not by day 15 (\n <jats:italic>P</jats:italic>\n = 0.30). CRP levels in the treatment group decreased at day 7 (5.53 ± 8.21 mg/L) compared to baseline but showed no significant difference from the control group (4.91 ± 12.54 mg/L,\n <jats:italic>P</jats:italic>\n = 0.98). At day 15, CRP levels remained lower in the treatment group (5.42 ± 10.41 mg/L) but increased in the control group (14.0 ± 37.16 mg/L), with no significant difference (\n <jats:italic>P</jats:italic>\n = 0.52). Ferritin levels dropped significantly in the treatment group by day 15 (from 560.58 ± 537.30 ng/mL to 127.51 ± 215.91 ng/mL) but increased in the control (\n <jats:italic>P</jats:italic>\n = 0.98). D-dimer levels decreased in the treatment group by day 15 but were not significantly different from controls (\n <jats:italic>P</jats:italic>\n = 0.56).\n </jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>These results indicate that while co-supplementation with AFO-202 and N-163 beta-glucans led to improvement in CRP, ferritin, and IL-6 levels in COVID-19 patients, only the reduction in IL-6 levels on day 7 reached statistical significance. Further long-term multicentric clinical research is warranted to validate the potential of these supplements as treatment adjuncts, for addressing inflammation in COVID-19, especially in vulnerable populations infected with emerging SARS-CoV-2 variants.</jats:p>\n </jats:sec>",
"alternative-id": [
"10.1177/27536130251327134"
],
"author": [
{
"affiliation": [
{
"name": "Department of Immunology, The Tamil Nadu Dr. M.G.R. Medical University, Chennai, India"
}
],
"family": "Pushkala",
"given": "Subramanian",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Department of Immunology, The Tamil Nadu Dr. M.G.R. Medical University, Chennai, India"
}
],
"family": "Seshayyan",
"given": "Sudha",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rajiv Gandhi Government General Hospital, Madras Medical College, Chennai, India"
}
],
"family": "Theranirajan",
"given": "Ethirajan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Rajiv Gandhi Government General Hospital, Madras Medical College, Chennai, India"
}
],
"family": "Sudhakar",
"given": "Doraisamy",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Paediatric Neurology, Jesuit Antonyraj Memorial Inter-disciplinary Centre for Advanced Recovery and Education (JAICARE), Madurai, India"
}
],
"family": "Raghavan",
"given": "Kadalraja",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Mary-Yoshio Translational Hexagon (MYTH), Nichi-In Centre for Regenerative Medicine (NCRM), Chennai, India"
}
],
"family": "Dedeepiya",
"given": "Vidyasagar Devaprasad",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medical Life Science, Kyushu University of Health and Welfare, Japan"
},
{
"name": "Institute of Immunology, Junsei Educational Institute, Nobeoka, Japan"
}
],
"family": "Ikewaki",
"given": "Nobunao",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Centre for Advancing Clinical Research (CACR), University of Yamanashi - School of Medicine, Chuo, Japan"
}
],
"family": "Iwasaki",
"given": "Masaru",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fujio-Eiji Academic Terrain (FEAT), Nichi-In Centre for Regenerative Medicine (NCRM), Chennai, India"
},
{
"name": "Haraguchi-Parikumar Advanced Remedies (HARP), SoulSynergy Ltd., Phoenix, Mauritius"
},
{
"name": "Cherian-Yoshii Translational Exemplary (CYTE), SoulSynergy Ltd., Phoenix, Mauritius"
}
],
"family": "Preethy",
"given": "Senthilkumar",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2646-2687",
"affiliation": [
{
"name": "Mary-Yoshio Translational Hexagon (MYTH), Nichi-In Centre for Regenerative Medicine (NCRM), Chennai, India"
},
{
"name": "Centre for Advancing Clinical Research (CACR), University of Yamanashi - School of Medicine, Chuo, Japan"
},
{
"name": "Fujio-Eiji Academic Terrain (FEAT), Nichi-In Centre for Regenerative Medicine (NCRM), Chennai, India"
},
{
"name": "Haraguchi-Parikumar Advanced Remedies (HARP), SoulSynergy Ltd., Phoenix, Mauritius"
},
{
"name": "Cherian-Yoshii Translational Exemplary (CYTE), SoulSynergy Ltd., Phoenix, Mauritius"
}
],
"authenticated-orcid": false,
"family": "Abraham",
"given": "Samuel JK",
"sequence": "additional"
}
],
"container-title": "Global Advances in Integrative Medicine and Health",
"container-title-short": "Global Advances in Integrative Medicine and Health",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"journals.sagepub.com"
]
},
"created": {
"date-parts": [
[
2025,
3,
14
]
],
"date-time": "2025-03-14T23:11:42Z",
"timestamp": 1741993902000
},
"deposited": {
"date-parts": [
[
2025,
3,
14
]
],
"date-time": "2025-03-14T23:11:44Z",
"timestamp": 1741993904000
},
"indexed": {
"date-parts": [
[
2025,
3,
15
]
],
"date-time": "2025-03-15T04:27:47Z",
"timestamp": 1742012867562,
"version": "3.38.0"
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2025,
1
]
]
},
"language": "en",
"license": [
{
"URL": "https://journals.sagepub.com/page/policies/text-and-data-mining-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
1,
1
]
],
"date-time": "2025-01-01T00:00:00Z",
"timestamp": 1735689600000
}
}
],
"link": [
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/27536130251327134",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/full-xml/10.1177/27536130251327134",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://journals.sagepub.com/doi/pdf/10.1177/27536130251327134",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "179",
"original-title": [],
"prefix": "10.1177",
"published": {
"date-parts": [
[
2025,
1
]
]
},
"published-online": {
"date-parts": [
[
2025,
3,
14
]
]
},
"published-print": {
"date-parts": [
[
2025,
1
]
]
},
"publisher": "SAGE Publications",
"reference": [
{
"DOI": "10.1186/s12879-021-05945-8",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_2_2"
},
{
"DOI": "10.1016/j.addr.2020.12.011",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_3_2"
},
{
"DOI": "10.1002/mnfr.201901071",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_4_2"
},
{
"DOI": "10.1155/2012/851362",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_5_2"
},
{
"DOI": "10.2478/helm-2018-0021",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_6_2"
},
{
"DOI": "10.3389/fimmu.2022.870632",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_7_2"
},
{
"DOI": "10.1016/j.clicom.2021.11.001",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_8_2"
},
{
"DOI": "10.1016/j.jceh.2022.06.008",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_9_2"
},
{
"DOI": "10.1007/s40200-022-01170-5",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_10_2"
},
{
"DOI": "10.1080/21645515.2021.1880210",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_11_2"
},
{
"DOI": "10.1186/s12959-020-00239-6",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_12_2"
},
{
"DOI": "10.1016/j.biopha.2021.112243",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_13_2"
},
{
"DOI": "10.26633/RPSP.2020.72",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_14_2"
},
{
"DOI": "10.1002/jcla.24881",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_15_2"
},
{
"article-title": "Beta 1,3-1,6 glucans produced by two novel strains of Aureobasidium pullulans exert immune and metabolic beneficial effects in healthy middle-aged Japanese men: results of an exploratory randomized control study",
"author": "Ikewaki N",
"first-page": "61",
"journal-title": "JAR Life",
"key": "e_1_3_6_16_2",
"unstructured": "Ikewaki N, Sonoda T, Kurosawa G, et al. Beta 1,3-1,6 glucans produced by two novel strains of Aureobasidium pullulans exert immune and metabolic beneficial effects in healthy middle-aged Japanese men: results of an exploratory randomized control study. JAR Life. 2023;12:61-71.",
"volume": "12",
"year": "2023"
},
{
"DOI": "10.3389/fimmu.2020.570993",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_17_2"
},
{
"DOI": "10.1155/2022/7424748",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_18_2"
},
{
"DOI": "10.3109/19390211.2013.859211",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_19_2"
},
{
"DOI": "10.1155/2012/895370",
"doi-asserted-by": "publisher",
"key": "e_1_3_6_20_2"
}
],
"reference-count": 19,
"references-count": 19,
"relation": {},
"resource": {
"primary": {
"URL": "https://journals.sagepub.com/doi/10.1177/27536130251327134"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Efficient Control of IL-6, CRP and Ferritin in COVID-19 Patients With Two Variants of Beta-1,3-1,6 Glucans in Combination: An Open-Label, Prospective, Randomised Clinical Trial",
"type": "journal-article",
"update-policy": "https://doi.org/10.1177/sage-journals-update-policy",
"volume": "14"
}