The CRISIS2 Study: A Phase 2, Randomized, Double Blind, Placebo-controlled, Multi-center Study Assessing the Safety and Anti-coronavirus Response of Suppression of Host Nucleotide Synthesis in Out-patient Adults With SARS-CoV-2
et al., NCT04575038, CRISIS2, NCT04575038, Apr 2021
RCT 115 outpatients in the USA, showing no significant differences with brequinar treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
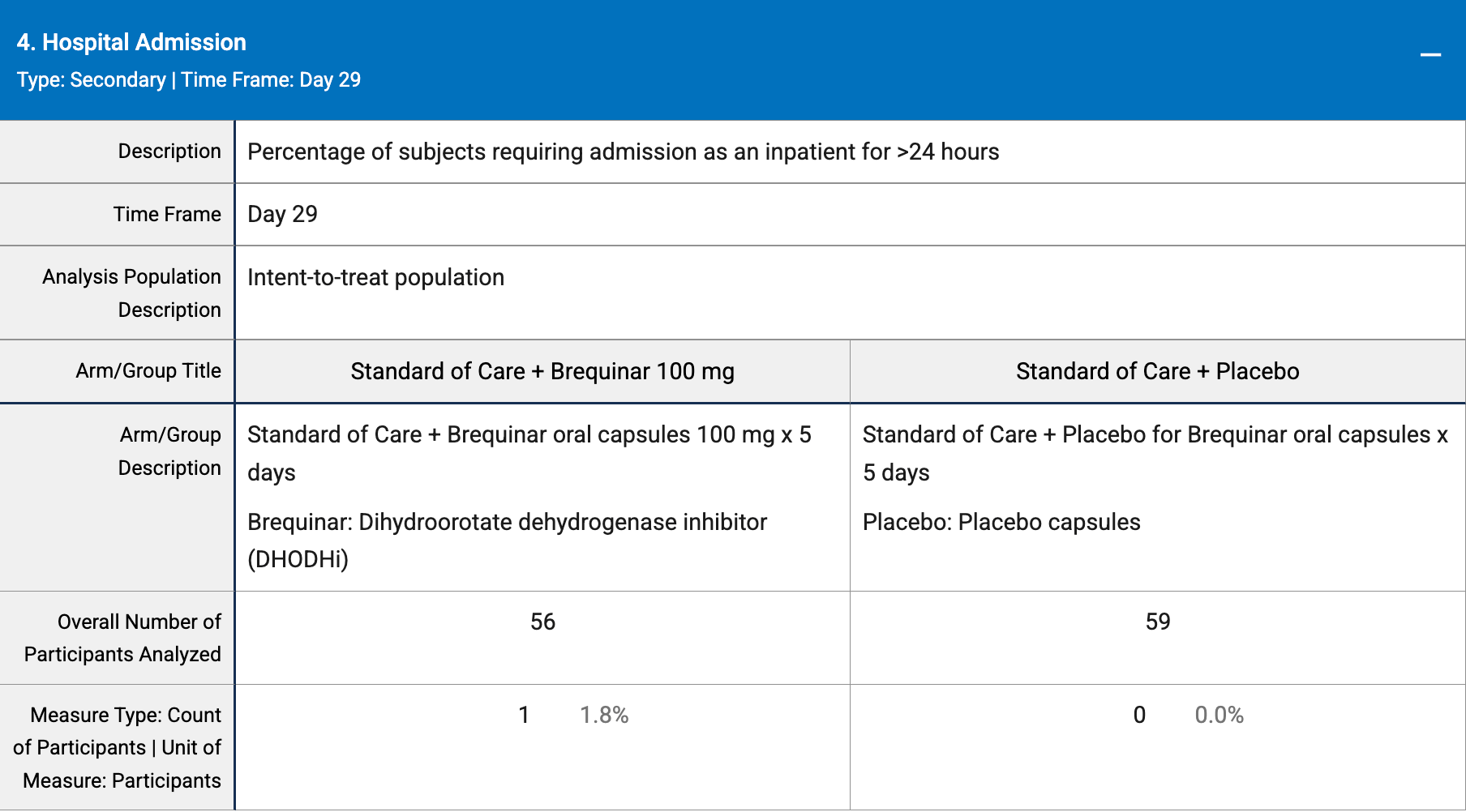
risk of hospitalization, 205.4% higher, RR 3.05, p = 0.49, treatment 1 of 56 (1.8%), control 0 of 59 (0.0%), continuity correction due to zero event (with reciprocal of the contrasting arm).
|
|
viral load, 33.9% higher, relative load 1.34, treatment 34, control 38, mid-recovery, day 8.
|
|
viral load, 7.9% lower, relative load 0.92, treatment 38, control 40, day 29.
|
|
viral load, 24.1% lower, relative load 0.76, treatment 36, control 39, day 15.
|
|
viral load, 13.9% higher, relative load 1.14, treatment 39, control 40, day 4.
|
|
time to viral-, 50.0% higher, relative time 1.50, treatment 43, control 45.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Powers et al., 28 Apr 2021, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04575038 (history) (CRISIS2).
Contact: clinical@clearcreekbio.com.