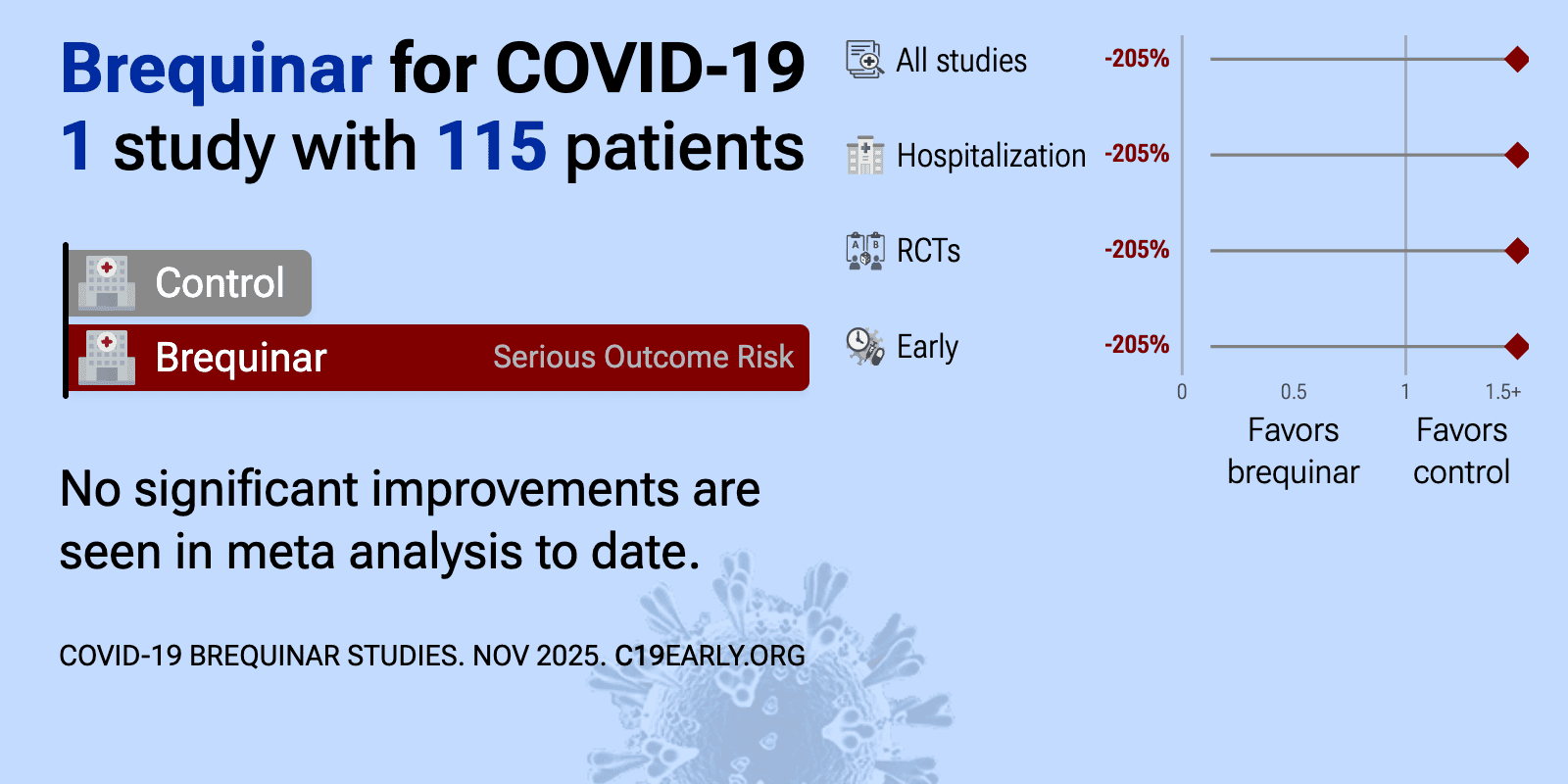
Brequinar is an orally available, small molecule, host-acting antiviral that inhibits the host enzyme dihydroorotate dehydrogenase (DHODH) to block de novo pyrimidine biosynthesis, potentially inhibiting SARS-CoV-2 replication.
Apr 28 2021 |
et al., NCT04575038 | The CRISIS2 Study: A Phase 2, Randomized, Double Blind, Placebo-controlled, Multi-center Study Assessing the Safety and Anti-coronavirus Response of Suppression of Host Nucleotide Synthesis in Out-patient Adults With SARS-CoV-2 |
| 205% higher hospitalization (p=0.49) and 34% worse viral clearance. RCT 115 outpatients in the USA, showing no significant differences with brequinar treatment. | ||