ODYSSEY: A Randomized, Double-blind, Placebo-controlled Study to Investigate the Efficacy of Tradipitant in Treating Inflammatory Lung Injury and Improving Clinical Outcomes Associated With Severe or Critical COVID-19 Infection
et al., NCT04326426, ODYSSEY, NCT04326426, Apr 2024
RCT 145 severe/critical COVID-19 patients in the USA, showing no significant difference with tradipitant treatment.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
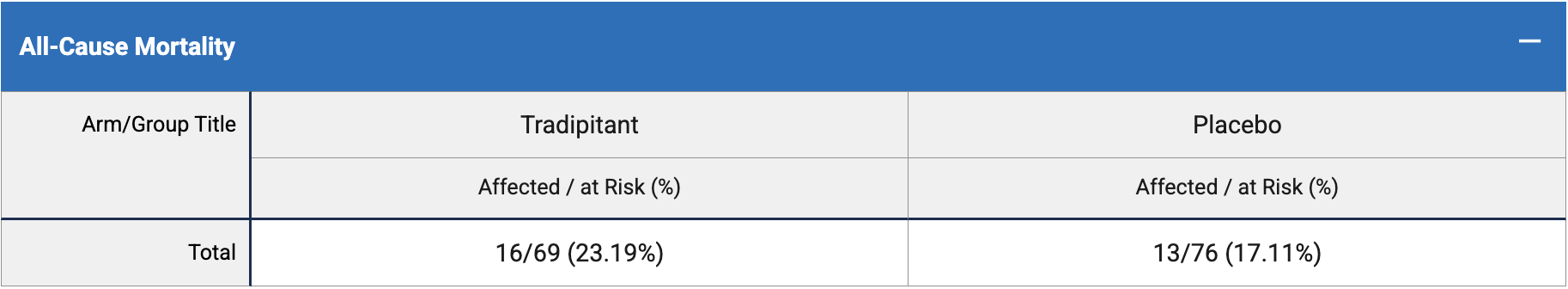
risk of death, 35.6% higher, RR 1.36, p = 0.41, treatment 16 of 69 (23.2%), control 13 of 76 (17.1%).
|
|
time to improvement, no change, relative time 1.00, treatment 69, control 76, time to 7-point scale improvement.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Polymeropoulos et al., 1 Apr 2024, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT04326426 (history) (ODYSSEY).
Tradipitant is an oral small-molecule neurokinin-1 receptor antagonist that blocks substance P signalling, potentially reducing neurogenic pulmonary inflammation.