Efficacy and safety of Ixekizumab vs. low-dose IL-2 vs. Colchicine vs. standard of care in the treatment of patients hospitalized with moderate-to-critical COVID-19: A pilot randomized clinical trial (STRUCK: Survival Trial Using Cytokine Inhibitors)
et al., Revista da Sociedade Brasileira de Medicina Tropical, doi:10.1590/0037-8682-0565-2022, STRUCK, NCT04724629, Apr 2022 (preprint)
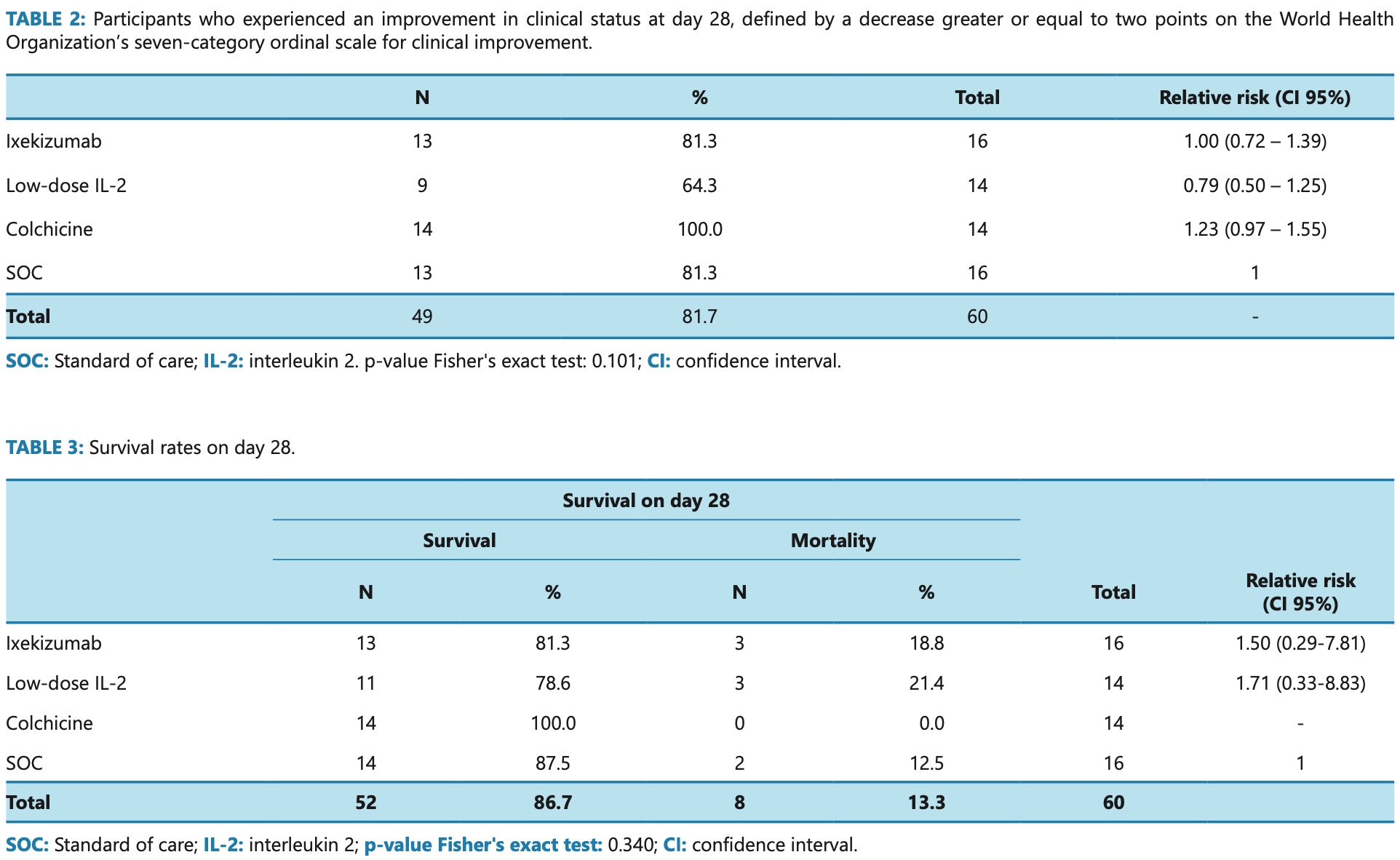
Open label RCT late stage hospitalized patients in Brazil showing no significant difference with IL-2 or ixekizumab treatment. Mortality was lower and recovery was greater with colchicine, without statistical significance.
Study covers interleukin-2, ixekizumab, and colchicine.
|
risk of death, 50.0% higher, RR 1.50, p = 1.00, treatment 3 of 16 (18.8%), control 2 of 16 (12.5%).
|
|
risk of no improvement, no change, RR 1.00, p = 1.00, treatment 3 of 16 (18.8%), control 3 of 16 (18.8%).
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pimenta Bonifácio et al., 28 Apr 2022, Randomized Controlled Trial, Brazil, peer-reviewed, mean age 48.9, 18 authors, study period 6 January, 2021 - 9 July, 2021, trial NCT04724629 (history) (STRUCK).
Contact: fbellissimo@usp.br, livia_pb@usp.br.
DOI record:
{
"DOI": "10.1590/0037-8682-0565-2022",
"ISSN": [
"1678-9849",
"0037-8682"
],
"URL": "http://dx.doi.org/10.1590/0037-8682-0565-2022",
"alternative-id": [
"S0037-86822023000100317"
],
"author": [
{
"ORCID": "http://orcid.org/0000-0002-4309-0304",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Bonifácio",
"given": "Lívia Pimenta",
"sequence": "first"
},
{
"ORCID": "http://orcid.org/0000-0002-5735-1333",
"affiliation": [
{
"name": "Science Valley Research Institute, Brasil; Grupo Leforte, Brasil"
}
],
"authenticated-orcid": false,
"family": "Ramacciotti",
"given": "Eduardo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9655-4734",
"affiliation": [],
"authenticated-orcid": false,
"family": "Agati",
"given": "Leandro Barile",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8232-5375",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Vilar",
"given": "Fernando Crivelenti",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-8672-4453",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Silva",
"given": "Anna Christina Tojal da",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2585-3870",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Louzada Júnior",
"given": "Paulo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3159-5687",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Fonseca",
"given": "Benedito Antônio Lopes da",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-7970-5403",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Souza",
"given": "Hayala Cristina Cavenague de",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0005-3588-3370",
"affiliation": [
{
"name": "Science Valley Research Institute, Brasil; Grupo Leforte, Brasil"
}
],
"authenticated-orcid": false,
"family": "Oliveira",
"given": "Caroline Candida Carvalho de",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0004-9950-0415",
"affiliation": [
{
"name": "Science Valley Research Institute, Brasil; Grupo Leforte, Brasil"
}
],
"authenticated-orcid": false,
"family": "Aguiar",
"given": "Valéria Cristina Resende",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0006-2098-0706",
"affiliation": [
{
"name": "Grupo Leforte, Brasil"
}
],
"authenticated-orcid": false,
"family": "Quadros",
"given": "Carlos Augusto de Aguiar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-6243-8855",
"affiliation": [
{
"name": "Hospital do Rocio, Brasil"
}
],
"authenticated-orcid": false,
"family": "Dusilek",
"given": "Cesar",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0001-9708-0294",
"affiliation": [
{
"name": "Hospital do Rocio, Brasil"
}
],
"authenticated-orcid": false,
"family": "Itinose",
"given": "Kengi",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0005-5583-6408",
"affiliation": [
{
"name": "Hospital do Rocio, Brasil"
}
],
"authenticated-orcid": false,
"family": "Risson",
"given": "Ricardo",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0009-0004-6049-7503",
"affiliation": [
{
"name": "Hospital do Rocio, Brasil"
}
],
"authenticated-orcid": false,
"family": "Ferreira",
"given": "Lucas Roberto Rivabem",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2999-4961",
"affiliation": [
{
"name": "Brazilian Clinical Research Institute, Brasil; Duke University Medical Center, USA"
}
],
"authenticated-orcid": false,
"family": "Lopes",
"given": "Renato Delascio",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-2026-6925",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Kallas",
"given": "Esper Georges",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0002-3736-7127",
"affiliation": [
{
"name": "Universidade de São Paulo, Brasil"
}
],
"authenticated-orcid": false,
"family": "Bellissimo-Rodrigues",
"given": "Fernando",
"sequence": "additional"
}
],
"container-title": "Revista da Sociedade Brasileira de Medicina Tropical",
"container-title-short": "Rev. Soc. Bras. Med. Trop.",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
14
]
],
"date-time": "2023-04-14T16:09:41Z",
"timestamp": 1681488581000
},
"deposited": {
"date-parts": [
[
2023,
4,
14
]
],
"date-time": "2023-04-14T16:10:01Z",
"timestamp": 1681488601000
},
"indexed": {
"date-parts": [
[
2023,
4,
15
]
],
"date-time": "2023-04-15T04:51:00Z",
"timestamp": 1681534260745
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023
]
]
},
"license": [
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "am",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
1,
1
]
],
"date-time": "2023-01-01T00:00:00Z",
"timestamp": 1672531200000
}
}
],
"link": [
{
"URL": "http://www.scielo.br/scielo.php?script=sci_pdf&pid=S0037-86822023000100317&tlng=en",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "530",
"original-title": [],
"prefix": "10.1590",
"published": {
"date-parts": [
[
2023
]
]
},
"published-online": {
"date-parts": [
[
2023
]
]
},
"publisher": "FapUNIFESP (SciELO)",
"reference": [
{
"key": "ref1",
"series-title": "COVID-19 Clinical management: living guidance",
"year": "2022"
},
{
"DOI": "10.1056/NEJMra2026131",
"article-title": "Cytokine Storm",
"author": "Fajgenbaum DC",
"doi-asserted-by": "crossref",
"first-page": "2255",
"issue": "23",
"journal-title": "N Engl J Med",
"key": "ref2",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.2174/0929867328666201209100259",
"article-title": "The Role of Interleukin-6 in the Pathogenesis, Prognosis and Treatment of Severe COVID-19",
"author": "Giannakodimos I",
"doi-asserted-by": "crossref",
"first-page": "5328",
"issue": "26",
"journal-title": "Curr Med Chem",
"key": "ref3",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1002/path.5642",
"article-title": "COVID-19: immunopathology, pathophysiological mechanisms, and treatment options",
"author": "van Eijk LE",
"doi-asserted-by": "crossref",
"first-page": "307",
"issue": "4",
"journal-title": "J Pathol",
"key": "ref4",
"volume": "254",
"year": "2021"
},
{
"DOI": "10.1177/11772719221106600",
"article-title": "Elevated Levels of Pleiotropic Interleukin-6 (IL-6) and Interleukin-10 (IL-10) are Critically Involved With the Severity and Mortality of COVID-19: An Updated Longitudinal Meta-Analysis and Systematic Review on 147 Studies",
"author": "Jafrin S",
"doi-asserted-by": "crossref",
"first-page": "11772719221106600",
"journal-title": "Biomark Insights",
"key": "ref5",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.1093/intimm/dxaa078",
"article-title": "IL-6 in inflammation, autoimmunity and cancer",
"author": "Hirano T",
"doi-asserted-by": "crossref",
"first-page": "127",
"issue": "3",
"journal-title": "Int Immunol",
"key": "ref6",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.789735",
"article-title": "Regulatory T Cells as Predictors of Clinical Course in Hospitalised COVID-19 Patients",
"author": "Caldrer S",
"doi-asserted-by": "crossref",
"first-page": "789735",
"journal-title": "Front Immunol",
"key": "ref7",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1371/journal.pone.0273186",
"article-title": "Excessive neutrophil recruitment promotes typical T-helper 17 responses in Coronavirus disease 2019 patients",
"author": "Choto TA",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "PloS One",
"key": "ref8",
"volume": "17",
"year": "2022"
},
{
"article-title": "IL-17 and IL-17-producing cells in protection versus pathology",
"author": "Mills KHG",
"journal-title": "Nat Rev Immunol",
"key": "ref9",
"year": "2022"
},
{
"DOI": "10.1111/sji.13131",
"article-title": "COVID-19 immunopathology with emphasis on Th17 response and cell-based immunomodulation therapy: Potential targets and challenges",
"author": "Pourgholaminejad A",
"doi-asserted-by": "crossref",
"issue": "2",
"journal-title": "Scand J Immunol",
"key": "ref10",
"volume": "95",
"year": "2022"
},
{
"DOI": "10.1002/jcp.30047",
"article-title": "Th17 and Treg cells function in SARS-CoV2 patients compared with healthy controls",
"author": "Sadeghi A",
"doi-asserted-by": "crossref",
"first-page": "2829",
"issue": "4",
"journal-title": "J Cell Physiol",
"key": "ref11",
"volume": "236",
"year": "2021"
},
{
"DOI": "10.1038/s41577-018-0046-y",
"article-title": "Biology and regulation of IL-2: from molecular mechanisms to human therapy",
"author": "Spolski R",
"doi-asserted-by": "crossref",
"first-page": "648",
"issue": "10",
"journal-title": "Nat Rev Immunol",
"key": "ref12",
"volume": "18",
"year": "2018"
},
{
"DOI": "10.3389/fimmu.2021.626235",
"article-title": "High Levels of Circulating IL-8 and Soluble IL-2R Are Associated With Prolonged Illness in Patients With Severe COVID-19",
"author": "Ma A",
"doi-asserted-by": "crossref",
"first-page": "626235",
"journal-title": "Front Immunol",
"key": "ref13",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1038/s41423-020-0484-x",
"article-title": "Potential contribution of increased soluble IL-2R to lymphopenia in COVID-19 patients",
"author": "Zhang Y",
"doi-asserted-by": "crossref",
"first-page": "878",
"issue": "8",
"journal-title": "Cell Mol Immunol",
"key": "ref14",
"volume": "17",
"year": "2020"
},
{
"DOI": "10.4049/jimmunol.1000142",
"article-title": "IL-17 enhancement of the IL-6 signaling cascade in astrocytes",
"author": "Ma X",
"doi-asserted-by": "crossref",
"first-page": "4898",
"issue": "9",
"journal-title": "J Immunol",
"key": "ref15",
"volume": "184",
"year": "2010"
},
{
"DOI": "10.1038/ni1439",
"article-title": "The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease",
"author": "Qian Y",
"doi-asserted-by": "crossref",
"first-page": "247",
"issue": "3",
"journal-title": "Nat Immunol",
"key": "ref16",
"volume": "8",
"year": "2007"
},
{
"DOI": "10.1016/j.isci.2021.102293",
"article-title": "ORF8 contributes to cytokine storm during SARS-CoV-2 infection by activating IL-17 pathway",
"author": "Lin X",
"doi-asserted-by": "crossref",
"first-page": "102293",
"issue": "4",
"journal-title": "iScience",
"key": "ref17",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.1152/ajplung.00344.2017",
"article-title": "IL-17 in the lung: the good, the bad, and the ugly",
"author": "Gurczynski SJ",
"doi-asserted-by": "crossref",
"first-page": "L6",
"issue": "1",
"journal-title": "Am J Physiol Lung Cell Mol Physiol",
"key": "ref18",
"volume": "314",
"year": "2018"
},
{
"DOI": "10.1371/journal.pone.0120912",
"article-title": "High peripheral blood th17 percent associated with poor lung function in cystic fibrosis",
"author": "Mulcahy EM",
"doi-asserted-by": "crossref",
"issue": "3",
"journal-title": "PloS One",
"key": "ref19",
"volume": "10",
"year": "2015"
},
{
"DOI": "10.4049/jimmunol.1100917",
"article-title": "IL-17 boosts proinflammatory outcome of antiviral response in human cells",
"author": "Ryzhakov G",
"doi-asserted-by": "crossref",
"first-page": "5357",
"issue": "10",
"journal-title": "J Immunol",
"key": "ref20",
"volume": "187",
"year": "2011"
},
{
"DOI": "10.1111/bcp.14437",
"article-title": "Potential role of IL-17 blocking agents in the treatment of severe COVID-19?",
"author": "Bulat V",
"doi-asserted-by": "crossref",
"first-page": "1578",
"issue": "3",
"journal-title": "Br J Clin Pharmacol",
"key": "ref21",
"volume": "87",
"year": "2021"
},
{
"DOI": "10.2147/JIR.S100940",
"article-title": "Generation and characterization of ixekizumab, a humanized monoclonal antibody that neutralizes interleukin-17A",
"author": "Liu L",
"doi-asserted-by": "crossref",
"first-page": "39",
"journal-title": "J Inflamm Res",
"key": "ref22",
"volume": "9",
"year": "2016"
},
{
"DOI": "10.1016/S2213-2600(21)00435-5",
"article-title": "Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial",
"doi-asserted-by": "crossref",
"first-page": "1419",
"issue": "12",
"journal-title": "Lancet Respir Med",
"key": "ref23",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17022",
"article-title": "Effect of Hydrocortisone on Mortality and Organ Support in Patients With Severe COVID-19: The REMAP-CAP COVID-19 Corticosteroid Domain Randomized Clinical Trial",
"author": "Angus DC",
"doi-asserted-by": "crossref",
"first-page": "1317",
"issue": "13",
"journal-title": "JAMA",
"key": "ref24",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0242318",
"article-title": "Colchicine reduces lung injury in experimental acute respiratory distress syndrome",
"author": "Dupuis J",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "PloS One",
"key": "ref25",
"volume": "15",
"year": "2020"
},
{
"DOI": "10.1136/rmdopen-2020-001455",
"article-title": "Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial",
"author": "Lopes MI",
"doi-asserted-by": "crossref",
"issue": "1",
"journal-title": "RMD Open",
"key": "ref26",
"volume": "7",
"year": "2021"
},
{
"DOI": "10.1038/nature02393",
"article-title": "Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain",
"author": "Ravelli RB",
"doi-asserted-by": "crossref",
"first-page": "198",
"issue": "6979",
"journal-title": "Nature",
"key": "ref27",
"volume": "428",
"year": "2004"
},
{
"DOI": "10.1016/S2213-2600(21)00222-8",
"article-title": "Colchicine for community-treated patients with COVID-19 (COLCORONA): a phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial",
"author": "Tardif JC",
"doi-asserted-by": "crossref",
"first-page": "924",
"issue": "8",
"journal-title": "Lancet Respir Med",
"key": "ref28",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1084/jem.20160417",
"article-title": "IL-6 promotes the differentiation of a subset of naive CD8+ T cells into IL-21-producing B helper CD8+ T cells",
"author": "Yang R",
"doi-asserted-by": "crossref",
"first-page": "2281",
"issue": "11",
"journal-title": "J Exp Med",
"key": "ref29",
"volume": "213",
"year": "2016"
},
{
"DOI": "10.1001/jamanetworkopen.2020.13136",
"article-title": "Effect of Colchicine vs Standard Care on Cardiac and Inflammatory Biomarkers and Clinical Outcomes in Patients Hospitalized With Coronavirus Disease 2019: The GRECCO-19 Randomized Clinical Trial",
"author": "Deftereos SG",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "JAMA Netw Open",
"key": "ref30",
"volume": "3",
"year": "2020"
},
{
"DOI": "10.1001/jamanetworkopen.2021.41328",
"article-title": "Effect of Colchicine vs Usual Care Alone on Intubation and 28-Day Mortality in Patients Hospitalized With COVID-19: A Randomized Clinical Trial",
"author": "Diaz R",
"doi-asserted-by": "crossref",
"issue": "12",
"journal-title": "JAMA Netw Open",
"key": "ref31",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1007/s11606-021-07203-8",
"article-title": "Colchicine Is Safe Though Ineffective in the Treatment of Severe COVID-19: a Randomized Clinical Trial (COLCHIVID)",
"author": "Absalon-Aguilar A",
"doi-asserted-by": "crossref",
"first-page": "4",
"issue": "1",
"journal-title": "J Gen Intern Med",
"key": "ref32",
"volume": "37",
"year": "2022"
},
{
"DOI": "10.18087/cardio.2021.2.n1560",
"article-title": "Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study",
"author": "Mareev VY",
"doi-asserted-by": "crossref",
"first-page": "15",
"issue": "2",
"journal-title": "Kardiologiia",
"key": "ref33",
"volume": "61",
"year": "2021"
},
{
"key": "ref34",
"series-title": "R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis",
"year": "2020"
},
{
"key": "ref35",
"series-title": "R: A language and environment for statistical computing. R Foundation for Statistical Computing",
"year": "2021"
},
{
"DOI": "10.2147/IJGM.S329810",
"article-title": "Colchicine in Recently Hospitalized Patients with COVID-19: A Randomized Controlled Trial (COL-COVID)",
"author": "Pascual-Figal DA",
"doi-asserted-by": "crossref",
"first-page": "5517",
"journal-title": "Int J Gen Med",
"key": "ref36",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1002/ptr.7319",
"article-title": "Efficacy and safety of colchicine treatment in patients with COVID-19: A prospective, multicenter, randomized clinical trial",
"author": "Pourdowlat G",
"doi-asserted-by": "crossref",
"first-page": "891",
"issue": "2",
"journal-title": "Phytother Res: PTR",
"key": "ref37",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1038/s41598-022-13424-6",
"article-title": "Efficacy of short-course colchicine treatment in hospitalized patients with moderate to severe COVID-19 pneumonia and hyperinflammation: a randomized clinical trial",
"author": "Cecconi A",
"doi-asserted-by": "crossref",
"first-page": "9208",
"issue": "1",
"journal-title": "Sci Rep",
"key": "ref38",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1684/ecn.2021.0463",
"article-title": "Interleukin 17 antagonist netakimab is effective and safe in the new coronavirus infection (COVID-19)",
"author": "Maslennikov R",
"doi-asserted-by": "crossref",
"first-page": "8",
"issue": "1",
"journal-title": "Eur Cytokine Netw",
"key": "ref39",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.1016/j.cyto.2021.155627",
"article-title": "Anti-IL-17 monoclonal antibodies in hospitalized patients with severe COVID-19: A pilot study",
"author": "Avdeev SN",
"doi-asserted-by": "crossref",
"first-page": "155627",
"journal-title": "Cytokine",
"key": "ref40",
"volume": "146",
"year": "2021"
},
{
"DOI": "10.1080/23744235.2022.2066171",
"article-title": "Blockade of interleukin seventeen (IL-17A) with secukinumab in hospitalized COVID-19 patients - the BISHOP study",
"author": "Resende GG",
"doi-asserted-by": "crossref",
"first-page": "591",
"issue": "8",
"journal-title": "Infect Dis",
"key": "ref41",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1038/s41584-019-0243-5",
"article-title": "Systemic effects of IL-17 in inflammatory arthritis",
"author": "Beringer A",
"doi-asserted-by": "crossref",
"first-page": "491",
"issue": "8",
"journal-title": "Nat Rev Rheumatol",
"key": "ref42",
"volume": "15",
"year": "2019"
},
{
"DOI": "10.1371/journal.pone.0273340",
"article-title": "Tocilizumab, netakimab, and baricitinib in patients with mild-to-moderate COVID-19: An observational study",
"author": "Bryushkova EA",
"doi-asserted-by": "crossref",
"issue": "8",
"journal-title": "PloS One",
"key": "ref43",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.3892/etm.2021.9658",
"article-title": "Recombinant interleukin-2 stimulates lymphocyte recovery in patients with severe COVID-19",
"author": "Zhu ME",
"doi-asserted-by": "crossref",
"first-page": "227",
"issue": "3",
"journal-title": "Exp Ther Med",
"key": "ref44",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"article-title": "Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses",
"doi-asserted-by": "crossref",
"first-page": "1941",
"issue": "10339",
"journal-title": "Lancet",
"key": "ref45",
"volume": "399",
"year": "2022"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0037-86822023000100317&tlng=en"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Infectious Diseases",
"Microbiology (medical)",
"Parasitology"
],
"subtitle": [],
"title": "Efficacy and safety of Ixekizumab vs. low-dose IL-2 vs. Colchicine vs. standard of care in the treatment of patients hospitalized with moderate-to-critical COVID-19: A pilot randomized clinical trial (STRUCK: Survival Trial Using Cytokine Inhibitors)",
"type": "journal-article",
"volume": "56"
}
pimentabonifacio2