An Exploratory, Randomized, Double-Blind Placebo-Controlled Study to Assess the Safety of an Anti-SARS-CoV-2 Monoclonal Antibody and Response to Treatment in Individuals With Long COVID (outSMART-LC)
et al., NCT05877508, outSMART-LC, NCT05877508, Oct 2025
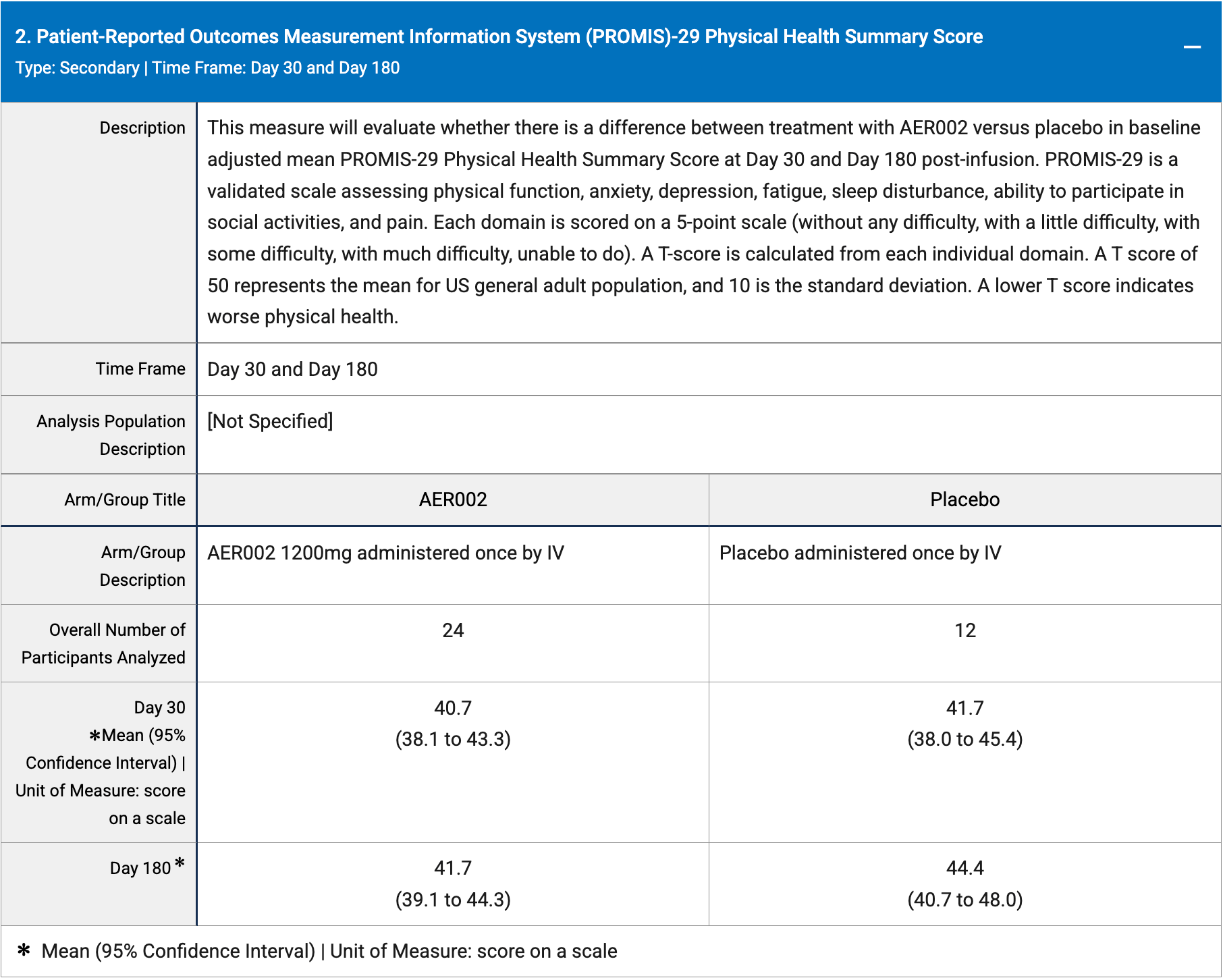
RCT 36 patients showing no improvement in long COVID with AER002.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of long COVID, 6.5% higher, RR 1.06, p = 0.25, treatment mean 41.7 (±6.5) n=24, control mean 44.4 (±6.45) n=12, relative PROMIS-29 score.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Peluso et al., 28 Oct 2025, Double Blind Randomized Controlled Trial, placebo-controlled, USA, preprint, 1 author, trial NCT05877508 (history) (outSMART-LC).
Contact: michael.peluso@ucsf.edu.