Nasopharyngeal Wash with Normal Saline Decreases SARS-CoV-2 Viral Load: A Randomized Pilot Controlled Trial
et al., Canadian Respiratory Journal, doi:10.1155/2022/8794127, NCT05525832, Sep 2022
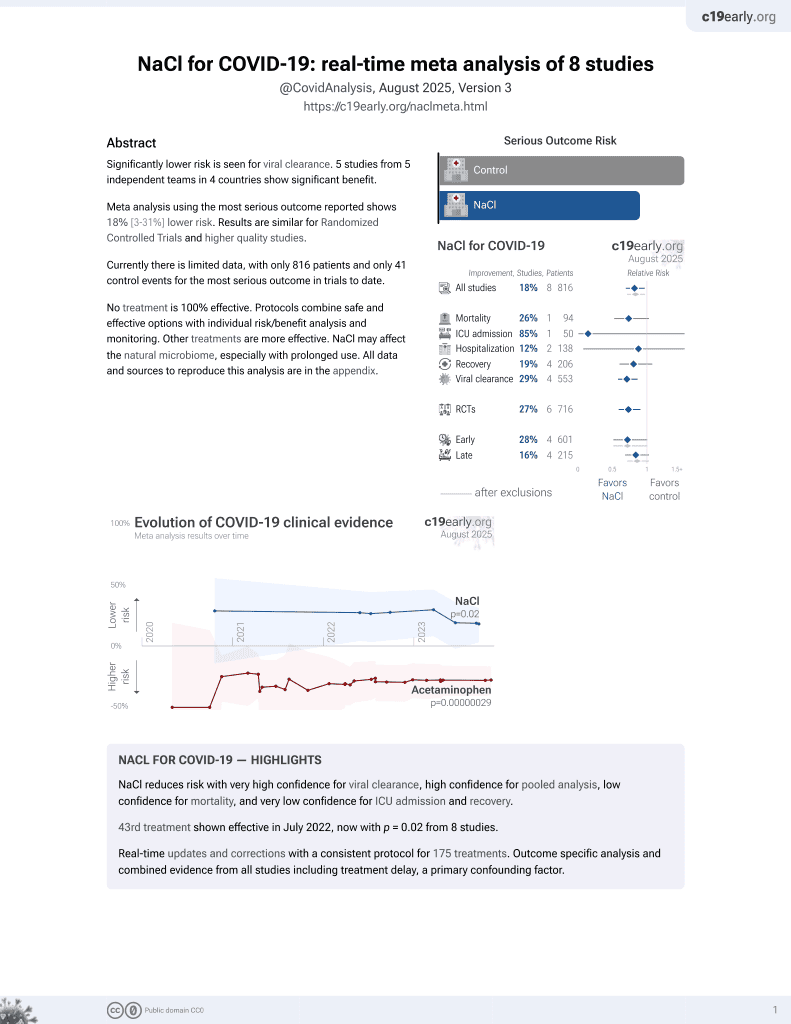
NaCl for COVID-19
44th treatment shown to reduce risk in
July 2022, now with p = 0.0028 from 9 studies.
Lower risk for progression and viral clearance.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
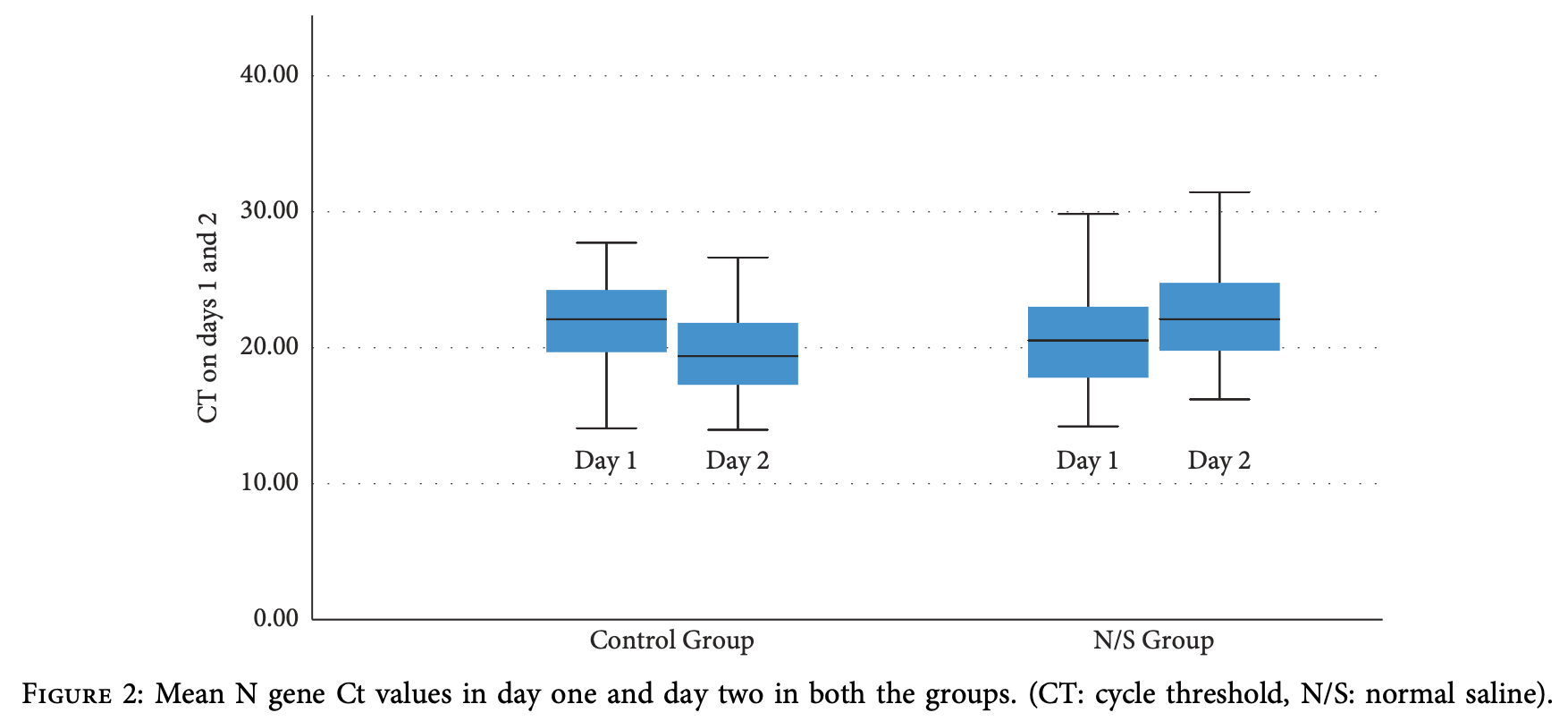
RCT 50 hospitalized COVID-19 pneumonia patients showing decreased SARS-CoV-2 viral load with nasopharyngeal washes using normal saline 0.9%. In the normal saline group, viral load decreased by 8.9% after 24 hours of treatment, while the control group experienced a 9.7% increase in viral load during the same period. The difference between groups was statistically significant (p=0.005). At follow-up two weeks after hospital discharge, significantly more patients in the normal saline group had negative COVID-19 tests compared to the control group (15 vs 6, p=0.02). No adverse events were reported with the nasal wash intervention. Patients in the control group were older (mean age 56 vs. 45).
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with significant baseline differences.
|
risk of ICU admission, 85.2% lower, RR 0.15, p = 0.24, treatment 0 of 24 (0.0%), control 3 of 26 (11.5%), NNT 8.7, relative risk is not 0 because of continuity correction due to zero events (with reciprocal of the contrasting arm).
|
|
risk of oxygen therapy, 63.9% lower, RR 0.36, p = 0.25, treatment 2 of 24 (8.3%), control 6 of 26 (23.1%), NNT 6.8, high flow nasal cannula or noninvasive ventilation.
|
|
risk of no viral clearance, 51.2% lower, RR 0.49, p = 0.009, treatment 9 of 24 (37.5%), control 20 of 26 (76.9%), NNT 2.5, negative test 2 weeks after discharge.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Pantazopoulos et al., 27 Sep 2022, Randomized Controlled Trial, Greece, peer-reviewed, mean age 51.0, 9 authors, study period 1 June, 2021 - 31 August, 2021, trial NCT05525832 (history).
Contact: pantazopoulosioannis@yahoo.com.
Nasopharyngeal Wash with Normal Saline Decreases SARS-CoV-2 Viral Load: A Randomized Pilot Controlled Trial
Canadian Respiratory Journal, doi:10.1155/2022/8794127
Background. Although great progress has been made over the past 2 years in the scientific understanding of the biology, epidemiology, and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), case morbidity and fatality rates remain a great concern and continue to challenge the healthcare resources worldwide as novel variants emerge. ere is therefore an urgent need for affordable and readily available strategies to reduce viral transmission. Previous studies in non-COVID-19 patients have demonstrated that administration of low-salt (isotonic but 0.0375% Na) and isotonic saline (0.9% Na) solutions has been associated with an immediate, significant reduction in the microbial antigens and a related decline of microbial burden. e aim of the present study was to determine the effect of nasal washes with normal saline 0.9% on nasopharyngeal viral load and outcome in hospitalized patients with COVID-19 pneumonia. Methods. We performed a prospective, randomized, pilot, controlled trial in 50 patients with confirmed COVID-19 disease. Patients were randomized into two groups, the normal saline group (received normal saline 0.9% solution for nasopharyngeal wash) and the control group (no treatment). In the normal saline group, nasopharyngeal wash was performed every 4 hours for a 16-hour period. Twenty-four hours after the baseline nasopharyngeal swab (and 8 hours after the last wash in the normal saline group), a second nasopharyngeal swab was collected for the semiquantitative estimation of the SARS-CoV-2 viral load as determined by cycle threshold (Ct) values. Results. In the normal saline group, mean N gene Ct values increased significantly 24 hours after the baseline measurement [ΔCt day2-day1 = 1.87 ± 3.11 cycles, p 0.007 (95% CI: 0.55 to 3.18)], indicating a decline in SARS-CoV-2 nasopharyngeal viral load by 8.9%. A significant decrease in mean N gene Ct values was observed in the control group, indicating an increase in viral load [ΔCt day2- day1 = -2.12 ± 2.66, p < 0.001 (95% CI: -3.20 to -1.05)] by 9.7%. e difference between the two groups 24 hours after admission and nasopharyngeal wash was 3.09 cycles (p 0.005, 95% CI: 0.97 to 5.20). Conclusion. Nasal washes with normal saline effectively decreased the viral load during hospitalization and at follow-up.
Disclosure *e work was supported solely by the authors and the payment for this publication will be made from their personal budget.
Conflicts of Interest *e authors declare that they have no conflicts of interest.
References
Bestle, Heindl, Limburg, TMPRSS2 and furin are both essential for proteolytic activation of SARS-CoV-2 in human airway cells, Life Science Alliance
Guy, Jackson, Acharya, Sturrock, Hooper et al., Angiotensin-converting enzyme-2 (ACE2): comparative modeling of the active site, specificity requirements, and chloride dependence, Biochemistry
Hasan, Paray, Hussain, A review on the cleavage priming of the spike protein on coronavirus by angiotensin-converting enzyme-2 and furin, Journal of Biomolecular Structure and Dynamics
Huijghebaert, Hoste, Vanham, Essentials in saline pharmacology for nasal or respiratory hygiene in times of COVID-19, European Journal of Clinical Pharmacology
Izidoro, Gouvea, Santos, A study of human furin specificity using synthetic peptides derived from natural substrates, and effects of potassium ions, Archives of Biochemistry and Biophysics
Jajou, Mutsaers-Van Oudheusden, Verweij, Rietveld, Murk, SARS-CoV-2 transmitters have more than three times higher viral loads than non-transmitters-practical use of viral load for disease control, Journal of Clinical Virology
Machado, Glaser, Araujo, Inhibition of severe acute respiratory syndrome coronavirus 2 replication by hypertonic saline solution in lung and kidney epithelial cells, ACS Pharmacology and Translational Science
Petersen, Ntoumi, Hui, Emergence of new SARS-CoV-2 variant of concern omicron (B.1.1.529)-highlights Africa's research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts, International Journal of Infectious Diseases
Poulas, First report of reduced severe acute respiratory syndrome coronavirus 2 viral load after nasopharyngeal wash with hypertonic water
Pujadas, Chaudhry, Mcbride, SARS-CoV-2 viral load predicts COVID-19 mortality, e Lancet Respiratory Medicine
Rabaan, Tirupathi, Sule, Viral dynamics and real-time RT-PCR Ct values correlation with disease severity in COVID-19, Diagnostics
Ramalingam, Cai, Wong, Antiviral innate immune response in non-myeloid cells is augmented by chloride ions via an increase in intracellular hypochlorous acid levels, Scientific Reports
Ramalingam, Graham, Dove, Morrice, Sheikh, A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold, Scientific Reports
Rosati, Giordano, Concato, Hypertonic saline nasal irrigation and gargling as an inexpensive practical adjunctive weapon to combat asymptomatic SARS-CoV-2 infections: a case report, Trends in Medicine
Tom, Mina, To interpret the SARS-CoV-2 test, consider the cycle threshold value, Clinical Infectious Diseases
Woods, Tan, Ullah, Frauenfelder, Ooi et al., *e effect of nasal irrigation formulation on the antimicrobial activity of nasal secretions, International Forum of Allergy & Rhinology
Zou, Ruan, Huang, SARS-CoV-2 viral load in upper respiratory specimens of infected patients, New England Journal of Medicine
DOI record:
{
"DOI": "10.1155/2022/8794127",
"ISSN": [
"1916-7245",
"1198-2241"
],
"URL": "http://dx.doi.org/10.1155/2022/8794127",
"abstract": "<jats:p>Background. Although great progress has been made over the past 2 years in the scientific understanding of the biology, epidemiology, and pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), case morbidity and fatality rates remain a great concern and continue to challenge the healthcare resources worldwide as novel variants emerge. There is therefore an urgent need for affordable and readily available strategies to reduce viral transmission. Previous studies in non-COVID-19 patients have demonstrated that administration of low-salt (isotonic but 0.0375% Na) and isotonic saline (0.9% Na) solutions has been associated with an immediate, significant reduction in the microbial antigens and a related decline of microbial burden. The aim of the present study was to determine the effect of nasal washes with normal saline 0.9% on nasopharyngeal viral load and outcome in hospitalized patients with COVID-19 pneumonia. Methods. We performed a prospective, randomized, pilot, controlled trial in 50 patients with confirmed COVID-19 disease. Patients were randomized into two groups, the normal saline group (received normal saline 0.9% solution for nasopharyngeal wash) and the control group (no treatment). In the normal saline group, nasopharyngeal wash was performed every 4 hours for a 16-hour period. Twenty-four hours after the baseline nasopharyngeal swab (and 8 hours after the last wash in the normal saline group), a second nasopharyngeal swab was collected for the semiquantitative estimation of the SARS-CoV-2 viral load as determined by cycle threshold (Ct) values. Results. In the normal saline group, mean N gene Ct values increased significantly 24 hours after the baseline measurement [ΔCtday2−day1 = 1.87 ± 3.11 cycles, <jats:inline-formula>\n <a:math xmlns:a=\"http://www.w3.org/1998/Math/MathML\" id=\"M1\">\n <a:mi>p</a:mi>\n <a:mo>=</a:mo>\n <a:mn>0.007</a:mn>\n </a:math>\n </jats:inline-formula> (95% CI: 0.55 to 3.18)], indicating a decline in SARS-CoV-2 nasopharyngeal viral load by 8.9%. A significant decrease in mean N gene Ct values was observed in the control group, indicating an increase in viral load [ΔCtday2-day1 = −2.12 ± 2.66, <jats:inline-formula>\n <c:math xmlns:c=\"http://www.w3.org/1998/Math/MathML\" id=\"M2\">\n <c:mi>p</c:mi>\n <c:mo><</c:mo>\n <c:mn>0.001</c:mn>\n </c:math>\n </jats:inline-formula> (95% CI: −3.20 to −1.05)] by 9.7%. The difference between the two groups 24 hours after admission and nasopharyngeal wash was 3.09 cycles (<jats:inline-formula>\n <e:math xmlns:e=\"http://www.w3.org/1998/Math/MathML\" id=\"M3\">\n <e:mi>p</e:mi>\n <e:mo>=</e:mo>\n <e:mn>0.005</e:mn>\n </e:math>\n </jats:inline-formula>, 95% CI: 0.97 to 5.20). Conclusion. Nasal washes with normal saline effectively decreased the viral load during hospitalization and at follow-up.</jats:p>",
"alternative-id": [
"8794127",
"8794127"
],
"author": [
{
"ORCID": "https://orcid.org/0000-0002-8846-519X",
"affiliation": [
{
"name": "Department of Emergency Medicine, Faculty of Medicine, University of Thessaly, Larissa, Greece"
},
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, Larissa, Greece"
}
],
"authenticated-orcid": true,
"family": "Pantazopoulos",
"given": "I.",
"sequence": "first"
},
{
"ORCID": "https://orcid.org/0000-0002-7634-4665",
"affiliation": [
{
"name": "Department of Anesthesiology, Faculty of Medicine, University of Thessaly, Larissa, Greece"
},
{
"name": "Outcomes Research Consortium, Cleveland, OH 44195, USA"
}
],
"authenticated-orcid": true,
"family": "Chalkias",
"given": "A.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-7595-5598",
"affiliation": [
{
"name": "Department of Emergency Medicine, Faculty of Medicine, University of Thessaly, Larissa, Greece"
}
],
"authenticated-orcid": true,
"family": "Mavrovounis",
"given": "G.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-6933-1068",
"affiliation": [
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, Larissa, Greece"
}
],
"authenticated-orcid": true,
"family": "Dimeas",
"given": "I.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-3653-260X",
"affiliation": [
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, Larissa, Greece"
}
],
"authenticated-orcid": true,
"family": "Sinis",
"given": "S.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-1065-5572",
"affiliation": [
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, Larissa, Greece"
}
],
"authenticated-orcid": true,
"family": "Miziou",
"given": "A.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0003-2885-4510",
"affiliation": [
{
"name": "Faculty of Nursing, University of Thessaly, Larissa, Greece"
}
],
"authenticated-orcid": true,
"family": "Rouka",
"given": "E.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-5397-1543",
"affiliation": [
{
"name": "Department of Pharmacy, University of Patras, Patras, Greece"
}
],
"authenticated-orcid": true,
"family": "Poulas",
"given": "K.",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0001-9541-1010",
"affiliation": [
{
"name": "Department of Respiratory Medicine, Faculty of Medicine, University of Thessaly, Larissa, Greece"
}
],
"authenticated-orcid": true,
"family": "Gourgoulianis",
"given": "K.",
"sequence": "additional"
}
],
"container-title": "Canadian Respiratory Journal",
"container-title-short": "Canadian Respiratory Journal",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
9,
27
]
],
"date-time": "2022-09-27T19:05:07Z",
"timestamp": 1664305507000
},
"deposited": {
"date-parts": [
[
2022,
10,
12
]
],
"date-time": "2022-10-12T14:57:27Z",
"timestamp": 1665586647000
},
"editor": [
{
"affiliation": [],
"family": "Qian",
"given": "Xin",
"sequence": "additional"
}
],
"indexed": {
"date-parts": [
[
2025,
5,
14
]
],
"date-time": "2025-05-14T02:51:23Z",
"timestamp": 1747191083690,
"version": "3.40.5"
},
"is-referenced-by-count": 6,
"issued": {
"date-parts": [
[
2022,
9,
27
]
]
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "unspecified",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
9,
27
]
],
"date-time": "2022-09-27T00:00:00Z",
"timestamp": 1664236800000
}
}
],
"link": [
{
"URL": "http://downloads.hindawi.com/journals/crj/2022/8794127.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/crj/2022/8794127.xml",
"content-type": "application/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "http://downloads.hindawi.com/journals/crj/2022/8794127.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "311",
"original-title": [],
"page": "1-6",
"prefix": "10.1155",
"published": {
"date-parts": [
[
2022,
9,
27
]
]
},
"published-print": {
"date-parts": [
[
2022,
9,
27
]
]
},
"publisher": "Wiley",
"reference": [
{
"article-title": "Emergence of new SARS-CoV-2 variant of concern omicron (B.1.1.529)—highlights Africa’s research capabilities, but exposes major knowledge gaps, inequities of vaccine distribution, inadequacies in global COVID-19 response and control efforts",
"author": "E. Petersen",
"first-page": "268",
"issue": "21",
"journal-title": "International Journal of Infectious Diseases",
"key": "1",
"volume": "114",
"year": "2021"
},
{
"DOI": "10.1056/NEJMc2001737",
"article-title": "SARS-CoV-2 viral load in upper respiratory specimens of infected patients",
"author": "L. Zou",
"doi-asserted-by": "crossref",
"first-page": "1177",
"issue": "12",
"journal-title": "New England Journal of Medicine",
"key": "2",
"volume": "382",
"year": "2020"
},
{
"DOI": "10.1016/j.jcv.2022.105131",
"doi-asserted-by": "publisher",
"key": "3"
},
{
"DOI": "10.1016/s2213-2600(20)30354-4",
"doi-asserted-by": "publisher",
"key": "4"
},
{
"DOI": "10.1038/s41598-018-37703-3",
"doi-asserted-by": "publisher",
"key": "5"
},
{
"article-title": "Information on COVID-19 treatment, prevention and research [Internet]",
"author": "COVID-19 Treatment Guidelines",
"key": "6",
"year": "2021"
},
{
"DOI": "10.3390/diagnostics11061091",
"doi-asserted-by": "publisher",
"key": "7"
},
{
"DOI": "10.1093/cid/ciaa619",
"doi-asserted-by": "publisher",
"key": "8"
},
{
"DOI": "10.1002/alr.21604",
"doi-asserted-by": "publisher",
"key": "9"
},
{
"DOI": "10.1021/bi035268s",
"doi-asserted-by": "publisher",
"key": "10"
},
{
"DOI": "10.1021/acsptsci.1c00080",
"doi-asserted-by": "publisher",
"key": "11"
},
{
"DOI": "10.1007/s00228-021-03102-3",
"doi-asserted-by": "publisher",
"key": "12"
},
{
"DOI": "10.1080/07391102.2020.1754293",
"doi-asserted-by": "publisher",
"key": "13"
},
{
"DOI": "10.26508/lsa.202000786",
"doi-asserted-by": "publisher",
"key": "14"
},
{
"DOI": "10.1016/j.abb.2009.05.013",
"doi-asserted-by": "publisher",
"key": "15"
},
{
"DOI": "10.1038/s41598-018-31936-y",
"doi-asserted-by": "publisher",
"key": "16"
},
{
"DOI": "10.15761/TiM.1000249",
"article-title": "Hypertonic saline nasal irrigation and gargling as an inexpensive practical adjunctive weapon to combat asymptomatic SARS-CoV-2 infections: a case report",
"author": "P. Rosati",
"doi-asserted-by": "crossref",
"issue": "6",
"journal-title": "Trends in Medicine",
"key": "17",
"volume": "20",
"year": "2020"
},
{
"article-title": "First report of reduced severe acute respiratory syndrome coronavirus 2 viral load after nasopharyngeal wash with hypertonic water",
"author": "K. Poulas",
"journal-title": "Qeios",
"key": "18",
"year": "2021"
}
],
"reference-count": 18,
"references-count": 18,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.hindawi.com/journals/crj/2022/8794127/"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Nasopharyngeal Wash with Normal Saline Decreases SARS-CoV-2 Viral Load: A Randomized Pilot Controlled Trial",
"type": "journal-article",
"volume": "2022"
}