Dexamethasone for Inpatients With COVID-19 in a National Cohort
et al., JAMA Network Open, doi:10.1001/jamanetworkopen.2023.8516, Apr 2023
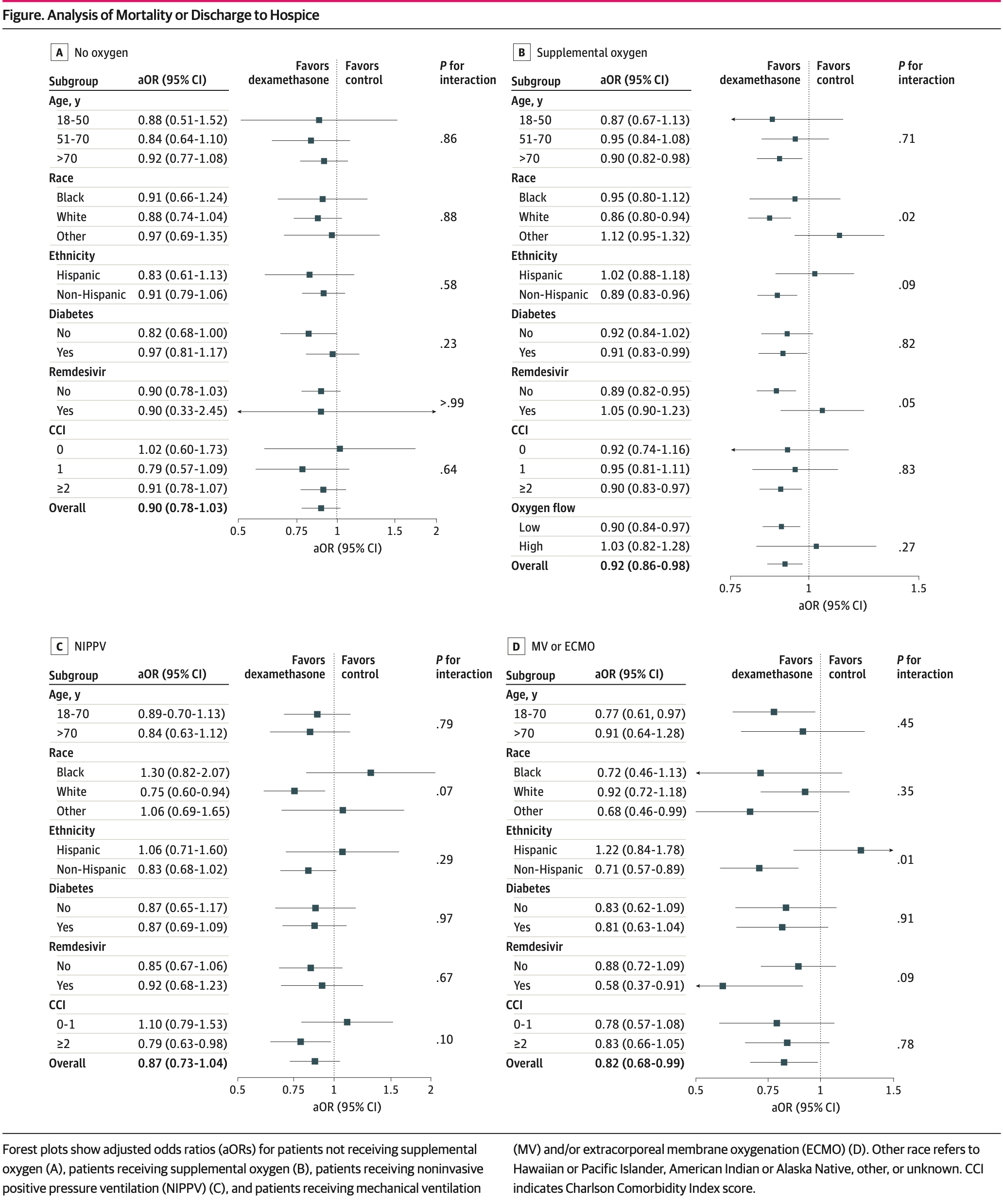
PSM retrospective 80,699 hospitalized COVID-19 patients showing reduced mortality or discharge to hospice with dexamethasone in patients requiring supplemental oxygen or mechanical ventilation/ECMO, but no significant difference in patients not requiring supplemental oxygen or on NIPPV.
Standard of Care (SOC) for COVID-19 in the study country,
the USA, is very poor with very low average efficacy for approved treatments1.
Only expensive, high-profit treatments were approved for early treatment. Low-cost treatments were excluded, reducing the probability of early treatment due to access and cost barriers, and eliminating complementary and synergistic benefits seen with many low-cost treatments.
|
risk of death, 9.6% lower, RR 0.90, p < 0.001, treatment 48,579, control 7,789, adjusted per study, all patients.
|
|
risk of death, 10.0% lower, OR 0.90, p = 0.14, treatment 7,537, control 5,503, adjusted per study, no oxygen, mortality or discharge to hospice, RR approximated with OR.
|
|
risk of death, 8.0% lower, OR 0.92, p = 0.01, treatment 48,579, control 7,789, adjusted per study, supplemental oxygen, mortality or discharge to hospice, RR approximated with OR.
|
|
risk of death, 13.0% lower, OR 0.87, p = 0.12, treatment 6,826, control 792, adjusted per study, NIPPV, mortality or discharge to hospice, RR approximated with OR.
|
|
risk of death, 18.0% lower, OR 0.82, p = 0.04, treatment 2,660, control 1,013, adjusted per study, MV/ECMO, mortality or discharge to hospice, RR approximated with OR.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mourad et al., 17 Apr 2023, retrospective, USA, peer-reviewed, median age 64.0, 7 authors, study period 1 July, 2020 - 31 October, 2021.
Dexamethasone for Inpatients With COVID-19 in a National Cohort
JAMA Network Open, doi:10.1001/jamanetworkopen.2023.8516
IMPORTANCE Limited effective therapeutics are available to hospitalized patients with COVID-19. Clinical trials and observational studies have shown varying effects of systemic corticosteroids, including dexamethasone, in hospitalized patients with COVID-19, with limited descriptions of important patient subgroups. OBJECTIVE To examine the clinical use of dexamethasone for hospitalized patients with COVID-19 respiratory illness and to explore the heterogeneity of treatment outcomes across different subgroups. DESIGN, SETTING, AND PARTICIPANTS This is a retrospective, propensity score-weighted cohort study of adult patients hospitalized for at least 48 hours for COVID-19 respiratory illness between July 1, 2020, and October 31, 2021, at a large health care network of 156 hospitals across the US. Data analysis was performed from March 2022 to February 2023. EXPOSURES Systemic dexamethasone administered within 48 hours of either admission or escalation in oxygen support. MAIN OUTCOMES AND MEASURES All-cause in-hospital mortality or discharge to hospice. RESULTS A total of 80 699 patients who met the eligibility criteria were identified (median [IQR] age, 64 [52-76] years; 37 606 women [46.6%]); 13 230 patients (16.4%) identified as Black, 49 222 (60.9%) as White, 18 247 (22.6%) as other race, and 20 340 (25.2%) as Hispanic ethnicity. Of these patients, 13 040 (16.2%) did not require supplemental oxygen within 48 hours of admission, 56 368 (69.8%) required supplemental oxygen, 7618 (9.4%) required noninvasive positive pressure ventilation (NIPPV), and 3673 (4.6%) required mechanical ventilation (MV) and/or extracorporeal membrane oxygenation (ECMO). After adjustment by propensity score overlap weighting, early use of dexamethasone was associated with reduction in a composite outcome of in-hospital mortality or discharge to hospice for patients receiving supplemental oxygen (aOR, 0.92; 95% CI, 0.86-0.98) and MV and/or ECMO (aOR, 0.82; 95% CI, 0.68-0.99). In contrast, all-cause inpatient mortality or discharge to hospice was not lower for patients who received dexamethasone in the no supplemental oxygen group (aOR, 0.90; 95% CI, 0.78-1.03) and in the NIPPV group (aOR, 0.87; 95% CI, 0.73-1.04). Importantly, patients with more comorbidities had greater benefit from dexamethasone use.
CONCLUSIONS AND RELEVANCE In this national multicenter cohort study of inpatients with COVID-19, early administration of dexamethasone was associated with significantly reduced odds of mortality or discharge to hospice in those requiring supplemental oxygen or MV and/or ECMO but not in those requiring no supplemental oxygen or NIPPV. These results support the continued use of systemic dexamethasone in patients hospitalized with COVID-19.
References
Ali, Azher, Baqi, Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial, CMAJ, doi:10.1503/cmaj.211698
Arabi, Mandourah, Hameed, Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome, Am J Respir Crit Care Med, doi:10.1164/rccm.201706-1172OC
Bigdelou, Sepand, Najafikhoshnoo, COVID-19 and preexisting comorbidities: risks, synergies, and clinical outcomes, Front Immunol, doi:10.3389/fimmu.2022.890517
Chaudhuri, Sasaki, Karkar, Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis, Intensive Care Med, doi:10.1007/s00134-021-06394-2
Crothers, Defaccio, Tate, Veterans Aging Cohort Study Clinical COVID-19 Working Group. Dexamethasone in hospitalised COVID-19 patients not on intensive respiratory support, Eur Respir J, doi:10.1183/13993003.02532-2021
Fried, Crawford, Mospan, Patient characteristics and outcomes of 11 721 patients with coronavirus disease 2019 (COVID-19) hospitalized across the United States, Clin Infect Dis, doi:10.1093/cid/ciaa1268
Ge, Li, Wu, Candido, Wei, Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: a population-based cohort study, PLoS One, doi:10.1371/journal.pone.0258154
Guan, Liang, Zhao, China Medical Treatment Expert Group for COVID-19. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis, Eur Respir J, doi:10.1183/13993003.00547-2020
Hca Healthcare, HCA Healthcare uses its COVID-19 registry by forming consortium with AHRQ and research institutions to accelerate COVID-19 research
Hca Healthcare, HCA factsheet
Horby, Lim, Emberson, Dexamethasone in hospitalized patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Kompaniyets, Pennington, Goodman, Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020, Prev Chronic Dis, doi:10.5888/pcd18.210123
Li, Thomas, Li, Addressing extreme propensity scores via the overlap weights, Am J Epidemiol, doi:10.1093/aje/kwy201
Magesh, John, Li, Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis, JAMA Netw Open, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jamanetworkopen.2021.34147&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2023.8516
Moreno, Rodríguez, Reyes, Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study, Intensive Care Med, doi:10.1007/s00134-018-5332-4
Richardson, Hirsch, Narasimhan, Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2020.6775&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2023.8516
Shaw, Yang, Mowery, Determinants of hospital outcomes for patients with COVID-19 in the University of Pennsylvania Health System, JAMA Network Open, doi:10.1371/journal.pone.0268528
Steinberg, Hudson, Goodman, ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome, N Engl J Med, doi:10.1056/NEJMoa051693
Sterne, Murthy, Diaz, WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2020.17023&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2023.8516
Thomas, Li, Pencina, Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial, JAMA, doi:https://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2020.7819&utm_campaign=articlePDF%26utm_medium=articlePDFlink%26utm_source=articlePDF%26utm_content=jamanetworkopen.2023.8516
Van Paassen, Vos, Hoekstra, Neumann, Boot et al., Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes, Crit Care, doi:10.1186/s13054-020-03400-9
Villar, Ferrando, Martínez, Dexamethasone in ARDS Network. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial, Lancet Respir Med, doi:10.1016/S2213-2600(19)30417-5
Yang, Liu, Zhou, Zhao, Zhao et al., The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis, J Infect, doi:10.1016/j.jinf.2020.03.062
Yang, Lorenzi, Papadogeorgou, Wojdyla, Li et al., Propensity score weighting for causal subgroup analysis, Stat Med, doi:10.1002/sim.9029
DOI record:
{
"DOI": "10.1001/jamanetworkopen.2023.8516",
"ISSN": [
"2574-3805"
],
"URL": "http://dx.doi.org/10.1001/jamanetworkopen.2023.8516",
"abstract": "<jats:sec id=\"ab-zoi230272-4\"><jats:title>Importance</jats:title><jats:p>Limited effective therapeutics are available to hospitalized patients with COVID-19. Clinical trials and observational studies have shown varying effects of systemic corticosteroids, including dexamethasone, in hospitalized patients with COVID-19, with limited descriptions of important patient subgroups.</jats:p></jats:sec><jats:sec id=\"ab-zoi230272-5\"><jats:title>Objective</jats:title><jats:p>To examine the clinical use of dexamethasone for hospitalized patients with COVID-19 respiratory illness and to explore the heterogeneity of treatment outcomes across different subgroups.</jats:p></jats:sec><jats:sec id=\"ab-zoi230272-6\"><jats:title>Design, Setting, and Participants</jats:title><jats:p>This is a retrospective, propensity score–weighted cohort study of adult patients hospitalized for at least 48 hours for COVID-19 respiratory illness between July 1, 2020, and October 31, 2021, at a large health care network of 156 hospitals across the US. Data analysis was performed from March 2022 to February 2023.</jats:p></jats:sec><jats:sec id=\"ab-zoi230272-7\"><jats:title>Exposures</jats:title><jats:p>Systemic dexamethasone administered within 48 hours of either admission or escalation in oxygen support.</jats:p></jats:sec><jats:sec id=\"ab-zoi230272-8\"><jats:title>Main Outcomes and Measures</jats:title><jats:p>All-cause in-hospital mortality or discharge to hospice.</jats:p></jats:sec><jats:sec id=\"ab-zoi230272-9\"><jats:title>Results</jats:title><jats:p>A total of 80 699 patients who met the eligibility criteria were identified (median [IQR] age, 64 [52-76] years; 37 606 women [46.6%]); 13 230 patients (16.4%) identified as Black, 49 222 (60.9%) as White, 18 247 (22.6%) as other race, and 20 340 (25.2%) as Hispanic ethnicity. Of these patients, 13 040 (16.2%) did not require supplemental oxygen within 48 hours of admission, 56 368 (69.8%) required supplemental oxygen, 7618 (9.4%) required noninvasive positive pressure ventilation (NIPPV), and 3673 (4.6%) required mechanical ventilation (MV) and/or extracorporeal membrane oxygenation (ECMO). After adjustment by propensity score overlap weighting, early use of dexamethasone was associated with reduction in a composite outcome of in-hospital mortality or discharge to hospice for patients receiving supplemental oxygen (aOR, 0.92; 95% CI, 0.86-0.98) and MV and/or ECMO (aOR, 0.82; 95% CI, 0.68-0.99). In contrast, all-cause inpatient mortality or discharge to hospice was not lower for patients who received dexamethasone in the no supplemental oxygen group (aOR, 0.90; 95% CI, 0.78-1.03) and in the NIPPV group (aOR, 0.87; 95% CI, 0.73-1.04). Importantly, patients with more comorbidities had greater benefit from dexamethasone use.</jats:p></jats:sec><jats:sec id=\"ab-zoi230272-10\"><jats:title>Conclusions and Relevance</jats:title><jats:p>In this national multicenter cohort study of inpatients with COVID-19, early administration of dexamethasone was associated with significantly reduced odds of mortality or discharge to hospice in those requiring supplemental oxygen or MV and/or ECMO but not in those requiring no supplemental oxygen or NIPPV. These results support the continued use of systemic dexamethasone in patients hospitalized with COVID-19.</jats:p></jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina"
}
],
"family": "Mourad",
"given": "Ahmad",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Durham, North Carolina"
}
],
"family": "Thibault",
"given": "Dylan",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Department of Medicine, Division of Infectious Diseases, Duke University Medical Center, Durham, North Carolina"
},
{
"name": "Duke Clinical Research Institute, Durham, North Carolina"
}
],
"family": "Holland",
"given": "Thomas L.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Meta Platforms, Inc, Seattle, Washington"
}
],
"family": "Yang",
"given": "Siyun",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Biobot Analytics, Cambridge, Massachusetts"
}
],
"family": "Young",
"given": "Allison R.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "HCA Healthcare Research Institute, Brentwood, Tennessee"
}
],
"family": "Arnold Egloff",
"given": "Shanna A.",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Duke Clinical Research Institute, Durham, North Carolina"
},
{
"name": "Department of Biostatistics and Bioinformatics, Duke University, Durham, North Carolina"
}
],
"family": "Thomas",
"given": "Laine E.",
"sequence": "additional"
}
],
"container-title": "JAMA Network Open",
"container-title-short": "JAMA Netw Open",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2023,
4,
17
]
],
"date-time": "2023-04-17T15:02:16Z",
"timestamp": 1681743736000
},
"deposited": {
"date-parts": [
[
2023,
4,
17
]
],
"date-time": "2023-04-17T15:02:22Z",
"timestamp": 1681743742000
},
"indexed": {
"date-parts": [
[
2025,
6,
4
]
],
"date-time": "2025-06-04T18:28:53Z",
"timestamp": 1749061733276
},
"is-referenced-by-count": 19,
"issue": "4",
"issued": {
"date-parts": [
[
2023,
4,
17
]
]
},
"journal-issue": {
"issue": "4",
"published-print": {
"date-parts": [
[
2023,
4,
3
]
]
}
},
"language": "en",
"link": [
{
"URL": "https://jamanetwork.com/journals/jamanetworkopen/articlepdf/2803926/mourad_2023_oi_230272_1680890824.28116.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "10",
"original-title": [],
"page": "e238516",
"prefix": "10.1001",
"published": {
"date-parts": [
[
2023,
4,
17
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
17
]
]
},
"publisher": "American Medical Association (AMA)",
"reference": [
{
"DOI": "10.1056/NEJMoa2021436",
"article-title": "Dexamethasone in hospitalized patients with Covid-19.",
"author": "Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med",
"key": "zoi230272r2",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.17023",
"article-title": "Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis.",
"author": "Sterne",
"doi-asserted-by": "publisher",
"first-page": "1330",
"issue": "13",
"journal-title": "JAMA",
"key": "zoi230272r3",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1007/s00134-021-06394-2",
"article-title": "Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis.",
"author": "Chaudhuri",
"doi-asserted-by": "publisher",
"first-page": "521",
"issue": "5",
"journal-title": "Intensive Care Med",
"key": "zoi230272r4",
"volume": "47",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03400-9",
"article-title": "Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes.",
"author": "van Paassen",
"doi-asserted-by": "publisher",
"first-page": "696",
"issue": "1",
"journal-title": "Crit Care",
"key": "zoi230272r5",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1268",
"article-title": "Patient characteristics and outcomes of 11 721 patients with coronavirus disease 2019 (COVID-19) hospitalized across the United States.",
"author": "Fried",
"doi-asserted-by": "publisher",
"first-page": "e558",
"issue": "10",
"journal-title": "Clin Infect Dis",
"key": "zoi230272r6",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1002/sim.v40.19",
"article-title": "Propensity score weighting for causal subgroup analysis.",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "4294",
"issue": "19",
"journal-title": "Stat Med",
"key": "zoi230272r9",
"volume": "40",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.7819",
"article-title": "Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial.",
"author": "Thomas",
"doi-asserted-by": "publisher",
"first-page": "2417",
"issue": "23",
"journal-title": "JAMA",
"key": "zoi230272r10",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1093/aje/kwy201",
"article-title": "Addressing extreme propensity scores via the overlap weights.",
"author": "Li",
"doi-asserted-by": "publisher",
"first-page": "250",
"issue": "1",
"journal-title": "Am J Epidemiol",
"key": "zoi230272r11",
"volume": "188",
"year": "2019"
},
{
"DOI": "10.1056/NEJMoa051693",
"article-title": "Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome.",
"author": "Steinberg",
"doi-asserted-by": "publisher",
"first-page": "1671",
"issue": "16",
"journal-title": "N Engl J Med",
"key": "zoi230272r12",
"volume": "354",
"year": "2006"
},
{
"DOI": "10.1016/S2213-2600(19)30417-5",
"article-title": "Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial.",
"author": "Villar",
"doi-asserted-by": "publisher",
"first-page": "267",
"issue": "3",
"journal-title": "Lancet Respir Med",
"key": "zoi230272r13",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1164/rccm.201706-1172OC",
"article-title": "Corticosteroid therapy for critically ill patients with Middle East Respiratory Syndrome.",
"author": "Arabi",
"doi-asserted-by": "publisher",
"first-page": "757",
"issue": "6",
"journal-title": "Am J Respir Crit Care Med",
"key": "zoi230272r14",
"volume": "197",
"year": "2018"
},
{
"DOI": "10.1016/j.jinf.2020.03.062",
"article-title": "The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis.",
"author": "Yang",
"doi-asserted-by": "publisher",
"first-page": "e13",
"issue": "1",
"journal-title": "J Infect",
"key": "zoi230272r15",
"volume": "81",
"year": "2020"
},
{
"DOI": "10.1007/s00134-018-5332-4",
"article-title": "Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study.",
"author": "Moreno",
"doi-asserted-by": "publisher",
"first-page": "1470",
"issue": "9",
"journal-title": "Intensive Care Med",
"key": "zoi230272r16",
"volume": "44",
"year": "2018"
},
{
"DOI": "10.1183/13993003.00547-2020",
"article-title": "Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis.",
"author": "Guan",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "Eur Respir J",
"key": "zoi230272r17",
"volume": "55",
"year": "2020"
},
{
"DOI": "10.1371/journal.pone.0258154",
"article-title": "Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: a population-based cohort study.",
"author": "Ge",
"doi-asserted-by": "publisher",
"issue": "10",
"journal-title": "PLoS One",
"key": "zoi230272r18",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2022.890517",
"article-title": "COVID-19 and preexisting comorbidities: risks, synergies, and clinical outcomes.",
"author": "Bigdelou",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "zoi230272r19",
"volume": "13",
"year": "2022"
},
{
"DOI": "10.5888/pcd18.210123",
"article-title": "Underlying medical conditions and severe illness among 540,667 adults hospitalized with COVID-19, March 2020-March 2021.",
"author": "Kompaniyets",
"doi-asserted-by": "publisher",
"journal-title": "Prev Chronic Dis",
"key": "zoi230272r20",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1001/jama.2020.6775",
"article-title": "Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area.",
"author": "Richardson",
"doi-asserted-by": "publisher",
"first-page": "2052",
"issue": "20",
"journal-title": "JAMA",
"key": "zoi230272r21",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(22)00519-0",
"article-title": "Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO Solidarity randomised trial and updated meta-analyses.",
"author": "WHO Solidarity Trial Consortium",
"doi-asserted-by": "publisher",
"first-page": "1941",
"issue": "10339",
"journal-title": "Lancet",
"key": "zoi230272r22",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.1503/cmaj.211698",
"article-title": "Remdesivir for the treatment of patients in hospital with COVID-19 in Canada: a randomized controlled trial.",
"author": "Ali",
"doi-asserted-by": "publisher",
"first-page": "E242",
"issue": "7",
"journal-title": "CMAJ",
"key": "zoi230272r23",
"volume": "194",
"year": "2022"
},
{
"DOI": "10.1001/jamanetworkopen.2021.34147",
"article-title": "Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis.",
"author": "Magesh",
"doi-asserted-by": "publisher",
"issue": "11",
"journal-title": "JAMA Netw Open",
"key": "zoi230272r24",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1183/13993003.02532-2021",
"article-title": "Dexamethasone in hospitalised COVID-19 patients not on intensive respiratory support.",
"author": "Crothers",
"doi-asserted-by": "publisher",
"issue": "1",
"journal-title": "Eur Respir J",
"key": "zoi230272r25",
"volume": "60",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0268528",
"article-title": "Determinants of hospital outcomes for patients with COVID-19 in the University of Pennsylvania Health System.",
"author": "Shaw",
"doi-asserted-by": "publisher",
"issue": "5",
"journal-title": "PLoS One",
"key": "zoi230272r26",
"volume": "17",
"year": "2022"
},
{
"DOI": "10.7326/M21-0857",
"article-title": "Use of hydroxychloroquine, remdesivir, and dexamethasone among adults hospitalized with COVID-19 in the United States: a retrospective cohort study.",
"author": "Mehta",
"doi-asserted-by": "publisher",
"first-page": "1395",
"issue": "10",
"journal-title": "Ann Intern Med",
"key": "zoi230272r27",
"volume": "174",
"year": "2021"
},
{
"key": "zoi230272r1",
"unstructured": "National Institutes of Health, COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. Accessed January 25, 2023. https://www.covid19treatmentguidelines.nih.gov/"
},
{
"key": "zoi230272r7",
"unstructured": "HCA Healthcare. HCA Healthcare uses its COVID-19 registry by forming consortium with AHRQ and research institutions to accelerate COVID-19 research. January 26, 2021. Accessed October 16, 2022. https://investor.hcahealthcare.com/news/news-details/2021/HCA-Healthcare-Uses-Its-COVID-19-Registry-by-Forming-Consortium-With-AHRQ-and-Research-Institutions-to-Accelerate-COVID-19-Research/default.aspx"
},
{
"key": "zoi230272r8",
"unstructured": "HCA Healthcare. HCA factsheet. June 2019. Accessed October 16, 2022. https://hcahealthcare.com/util/forms/press-kit/HCA-presskit-fact-sheet-a.pdf"
},
{
"key": "zoi230272r28",
"unstructured": "US Centers for Disease Control and Prevention. COVID data tracker. Accessed July 16, 2022. https://covid.cdc.gov/covid-data-tracker"
}
],
"reference-count": 28,
"references-count": 28,
"relation": {},
"resource": {
"primary": {
"URL": "https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2803926"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Dexamethasone for Inpatients With COVID-19 in a National Cohort",
"type": "journal-article",
"volume": "6"
}