NBS superfood: a promising adjunctive therapy in critically ill ICU patients with omicron variant of COVID-19
et al., AMB Express, doi:10.1186/s13568-024-01690-8, IRCT20230116057135N2, Mar 2024
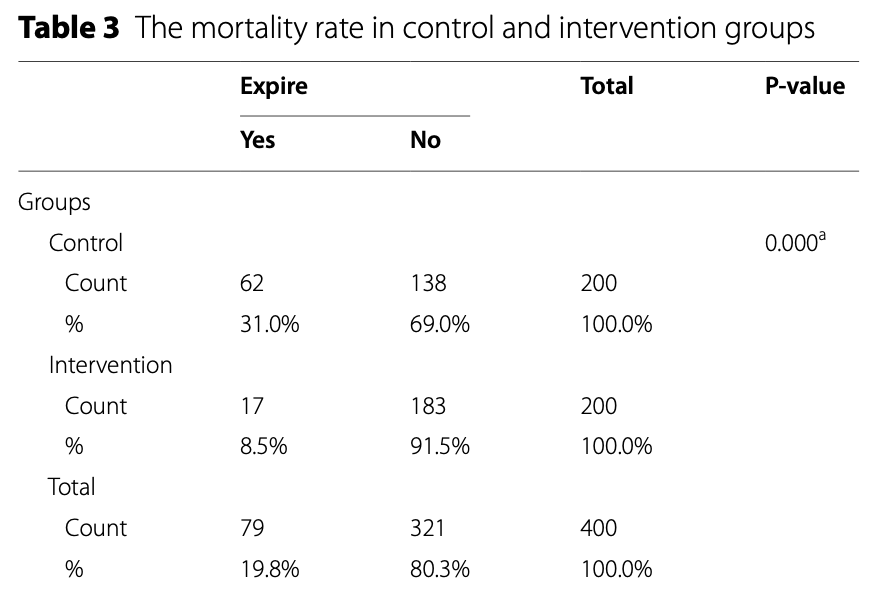
RCT 400 critically ill ICU patients with omicron-related ARDS showing significantly reduced mortality and inflammatory markers with a nutritional supplement containing vitamins A, B1-B3, B5, B6, B9, C, D, K, and zinc, potassium, manganese, magnesium, phosphorus, sulfur, boron, calcium, iron, copper, and other ingredients. The treatment group had significantly lower levels of CRP, ESR, D-Dimer, and CPK, and higher lymphocyte counts compared to the control group after 14 days. Mortality was 8.5% in the treatment group vs. 31% in the control group (p=0.001).
This study is excluded in meta-analysis:
many combined treatments which may significantly contribute to the effect seen.
|
risk of death, 72.6% lower, RR 0.27, p < 0.001, treatment 17 of 200 (8.5%), control 62 of 200 (31.0%), NNT 4.4.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Mosadegh et al., 24 Mar 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Iran, peer-reviewed, 12 authors, this trial uses multiple treatments in the treatment arm (combined with multiple vitamins and minerals) - results of individual treatments may vary, trial IRCT20230116057135N2.
Contact: m-mosadegh@alumnus.tums.ac.ir.
NBS superfood: a promising adjunctive therapy in critically ill ICU patients with omicron variant of COVID-19
AMB Express, doi:10.1186/s13568-024-01690-8
This clinical trial aimed to assess the impact of Nutrition Bio-shield superfood (NBS) on clinical status among critically ill ICU patients suffering from acute respiratory distress syndrome (ARDS) due to the Omicron variant of COVID-19. A total of 400 patients with confirmed Omicron-related ARDS were randomly assigned to either the intervention group (n = 200) or the control group (n = 200). Patients in the intervention group received 1.5 g of NBS powder daily for 2 weeks in addition to standard antiviral treatment, while the control group received a placebo alongside standard antiviral therapy. Serum samples were collected from all patients in both groups, and various clinical and laboratory parameters, including ESR, CRP, D-Dimer, CPK, WBC count, lymphocyte count, and lymphocyte percentage, were measured using established methodologies. Following a 14-day intervention period, the intervention group exhibited a significant reduction in mean serum levels of CRP (15.39 vs. 48.49; P < 0.001), ESR (14.28 vs. 34.03; P < 0.001), D-Dimer (485.18 vs. 1009.13; P = 0.001), and CPK (68.93 vs. 131.48; P < 0.001) compared to the control group. Conversely, a significant increase was observed in the mean serum levels of lymphocytes (1537.06 vs. 1152.60; P < 0.001) in the intervention group after 14 days of treatment compared to the control group. The remarkable reduction in inflammatory markers and mortality rates observed with NBS supplementation alongside standard antiviral treatment underscores its crucial role in mitigating inflammation and achieving an important milestone in the fight against COVID-19.
Author contributions M. M., A. K. and Y. E.: conceptualization; data curation; formal analysis; and writing-original draft. Y. E., M. N., S. H. H., F. A. J.: conceptualization; methodology; project administration; and writing-original draft. M. M., E. A., R. A., G. K.: data curation; formal analysis; writing-original draft; and writing-review & editing. F. A. J., N. A., S. M.: language editing.
Declarations Ethics approval and consent to participate The study protocols adhered to the principles outlined in the Helsinki Declaration, with all procedures receiving official validation from the Ethics Commit-
Consent for publication Not applicable.
Competing interests All of the authors declare that there are no commercial, personal, political, or any other potential conflicting interests related to the submitted manuscript.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ahmed, An overview of inflammation: mechanism and consequences, Front Biol
Askari, Alikiaii, Soleimani, Sahebkar, Mirjalili et al., Effect of curcumin-pipeine supplementation on clinical status, mortality rate, oxidative stress, and inflammatory markers in critically ill ICU patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial, Trials
Azimi, Hamidi-Farahani, Asgari, Rajabi, Ahmadi et al., Molecular detection and clinical characteristics of bacterial and viral main etiological agents causing respiratory tract infections in Tehran, Iran, Gene Rep
Bayat, Khalkhali, Mahjoub, Nutrition bio-shield superfood: healthy and live herbal supplement for immune system enhancement, Int J Nutr Food Eng
Beigmohammadi, Bitarafan, Hoseindokht, Abdollahi, Amoozadeh et al., Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial, Trials
Beigmohammadi, Bitarafan, Hoseindokht, Abdollahi, Amoozadeh et al., The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial, Trials
Emadi, Chua, Talwani, Bentzen, Baddley, Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: a structured summary of a study protocol for a randomised controlled trial, Trials
Fan, Li, Zhang, Wan, Zhang et al., SARS-CoV-2 Omicron variant: recent progress and future perspectives, Signal Transduct Target Ther
Gadotti, De Castro Deus, Telles, Wind, Goes et al., IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection, Virus Res
Gönen, Alaylıoğlu, Durcan, Özdemir, Şahin et al., Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1, Nutrients
Jafarzadeh, Jafarzadeh, Nozari, Mokhtari, Nemati, Lymphopenia an important immunological abnormality in patients with COVID-19: possible mechanisms, Scand J Immunol
Jalilian, Keshavarz, Khazaei, Nezamdoost, Hashemi et al., The effects of nutrition bio-shield superfood powder on immune system function: a clinical trial study among patients with COVID-19, Front Immunol
Kandeel, Mohamed, El-Lateef, Venugopala, El-Beltagi, Omicron variant genome evolution and phylogenetics, J Med Virol
Kuwahara, Horie, Ishikawa, Tsuda, Kawakami et al., Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy, Free Radical Biol Med
Le, Andreadakis, Kumar, Román, Tollefsen et al., The COVID-19 vaccine development landscape, Nat Rev Drug Discov
Legacy, Seely, Conte, Psihogios, Ramsay et al., Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: study protocol for a randomised, double-blind, placebo controlled clinical trial, BMJ Open
Leulseged, Hassen, Ayele, Tsegay, Abebe et al., Laboratory biomarkers of COVID-19 disease severity and outcome: findings from a developing country, PLoS ONE
Mehta, Mcauley, Brown, Sanchez, Tattersall et al., COVID-19: consider cytokine storm syndromes and immunosuppression, The Lancet
Meo, Meo, Al-Jassir, Klonoff, Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics, Eur Rev Med Pharmacol Sci
Mortaz, Tabarsi, Jamaati, Roofchayee, Dezfuli et al., Increased serum levels of soluble TNF-α receptor is associated with ICU mortality in COVID-19 patients, Front Immunol
Mosadegh, Khalkhali, Erfani, Nezamdoost, The effect of Nutrition Bio-shield superfood (NBS) on disease severity and laboratory biomarkers in patients with COVID-19: a randomized clinical trial, Microb Pathog
Mosadegh, Khalkhali, Sadeghi, Erfani, The anti-inflammatory effects of the nutrition bio-shield (NBS) supplement intake on adjuvantinduced rheumatoid arthritis in rat, Turk J Immunol
Oristrell, Oliva, Casado, Subirana, Domínguez et al., Vitamin D supplementation and COVID-19 risk: a population-based, cohort study, J Endocrinol Invest
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature
Ruan, Yang, Wang, Jiang, Song, Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China, Intensive Care Med
Shanafelt, Kay, Jenkins, Call, Zent et al., B-cell count and survival: differentiating chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis based on clinical outcome, Blood J Am Soc Hematol
Speakman, Michienzi, Badowski, Vitamins, supplements and COVID-19: a review of currently available evidence, Drugs in Context
Sproston, Ashworth, Role of C-reactive protein at sites of inflammation and infection, Front Immunol
Tegally, Wilkinson, Giovanetti, Iranzadeh, Fonseca et al., Detection of a SARS-CoV-2 variant of concern in South Africa, Nature
Tzenios, Chahine, Tazanios, Better strategies for coronavirus (COVID-19) vaccination, Special J Med Acad Other Life Sci, doi:10.58676/sjmas.v1i2.11
Wang, Nair, Liu, Iketani, Luo et al., Antibody resistance of SARS-CoV-2 variants B. 1.351 and B. 1.1. 7, Nature
Weidmann, Ofori, Rai, Laboratory biomarkers in the management of patients with COVID-19, Am J Clin Pathol
Yao, Cao, Wang, Shi, Liu et al., D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study, J Intensive Care
Zhou, Yu, Du, Fan, Liu et al., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, Lancet
DOI record:
{
"DOI": "10.1186/s13568-024-01690-8",
"ISSN": [
"2191-0855"
],
"URL": "http://dx.doi.org/10.1186/s13568-024-01690-8",
"abstract": "<jats:title>Abstract</jats:title><jats:p>This clinical trial aimed to assess the impact of Nutrition Bio-shield superfood (NBS) on clinical status among critically ill ICU patients suffering from acute respiratory distress syndrome (ARDS) due to the Omicron variant of COVID-19. A total of 400 patients with confirmed Omicron-related ARDS were randomly assigned to either the intervention group (n = 200) or the control group (n = 200). Patients in the intervention group received 1.5 g of NBS powder daily for 2 weeks in addition to standard antiviral treatment, while the control group received a placebo alongside standard antiviral therapy. Serum samples were collected from all patients in both groups, and various clinical and laboratory parameters, including ESR, CRP, D-Dimer, CPK, WBC count, lymphocyte count, and lymphocyte percentage, were measured using established methodologies. Following a 14-day intervention period, the intervention group exhibited a significant reduction in mean serum levels of CRP (15.39 vs. 48.49; P < 0.001), ESR (14.28 vs. 34.03; P < 0.001), D-Dimer (485.18 vs. 1009.13; P = 0.001), and CPK (68.93 vs. 131.48; P < 0.001) compared to the control group. Conversely, a significant increase was observed in the mean serum levels of lymphocytes (1537.06 vs. 1152.60; P < 0.001) in the intervention group after 14 days of treatment compared to the control group. The remarkable reduction in inflammatory markers and mortality rates observed with NBS supplementation alongside standard antiviral treatment underscores its crucial role in mitigating inflammation and achieving an important milestone in the fight against COVID-19.</jats:p>",
"alternative-id": [
"1690"
],
"article-number": "33",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "6 January 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "12 March 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "24 March 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The study protocols adhered to the principles outlined in the Helsinki Declaration, with all procedures receiving official validation from the Ethics Committee of Hamadan University of Medical Sciences (Protocol Number: IR.UMSHA.REC.1401.1043; Approval Date: 2023-03-04). Additionally, the study’s protocol has been registered in the Iranian Registry of Clinical Trials (IRCT), accessible at ExternalRef removed (IRCT Registration Number: IRCT20230116057135N2; Registration Date: 2023-04-05). The aims of the study were explained to patients. A questionnaire was prepared for each of the patients and a written informed consent was acquired from all patients."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "All of the authors declare that there are no commercial, personal, political, or any other potential conflicting interests related to the submitted manuscript."
}
],
"author": [
{
"affiliation": [],
"family": "Mosadegh",
"given": "Mehrdad",
"sequence": "first"
},
{
"affiliation": [],
"family": "Khalkhali",
"given": "Aref",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Erfani",
"given": "Yousef",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nezamdoost",
"given": "Manije",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hashemi",
"given": "Seyyed Hamid",
"sequence": "additional"
},
{
"ORCID": "http://orcid.org/0000-0003-3134-1441",
"affiliation": [],
"authenticated-orcid": false,
"family": "Azizi Jalilian",
"given": "Farid",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ansari",
"given": "Nastaran",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mahmoudvand",
"given": "Shahab",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mamani",
"given": "Mojgan",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Abdoli",
"given": "Elham",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Amini",
"given": "Razieh",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Kalvandi",
"given": "Gholamreza",
"sequence": "additional"
}
],
"container-title": "AMB Express",
"container-title-short": "AMB Expr",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
3,
24
]
],
"date-time": "2024-03-24T13:01:33Z",
"timestamp": 1711285293000
},
"deposited": {
"date-parts": [
[
2024,
3,
24
]
],
"date-time": "2024-03-24T13:01:43Z",
"timestamp": 1711285303000
},
"indexed": {
"date-parts": [
[
2024,
3,
24
]
],
"date-time": "2024-03-24T13:44:34Z",
"timestamp": 1711287874643
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
3,
24
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
24
]
],
"date-time": "2024-03-24T00:00:00Z",
"timestamp": 1711238400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
3,
24
]
],
"date-time": "2024-03-24T00:00:00Z",
"timestamp": 1711238400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13568-024-01690-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s13568-024-01690-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s13568-024-01690-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
3,
24
]
]
},
"published-online": {
"date-parts": [
[
2024,
3,
24
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1007/s11515-011-1123-9",
"author": "AU Ahmed",
"doi-asserted-by": "publisher",
"first-page": "274",
"issue": "4",
"journal-title": "Front Biol",
"key": "1690_CR1",
"unstructured": "Ahmed AU (2011) An overview of inflammation: mechanism and consequences. Front Biol 6(4):274–281",
"volume": "6",
"year": "2011"
},
{
"DOI": "10.1186/s13063-021-05372-9",
"author": "G Askari",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Trials",
"key": "1690_CR2",
"unstructured": "Askari G, Alikiaii B, Soleimani D, Sahebkar A, Mirjalili M, Feizi A, Iraj B, Bagherniya M (2021) Effect of curcumin-pipeine supplementation on clinical status, mortality rate, oxidative stress, and inflammatory markers in critically ill ICU patients with COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials 22(1):1–3",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1016/j.genrep.2021.101267",
"author": "T Azimi",
"doi-asserted-by": "publisher",
"journal-title": "Gene Rep",
"key": "1690_CR3",
"unstructured": "Azimi T, Hamidi-Farahani R, Asgari A, Rajabi J, Ahmadi M, Darvishi M, Aminianfar M, Naghoosi H, Soleiman-Meigooni S (2021) Molecular detection and clinical characteristics of bacterial and viral main etiological agents causing respiratory tract infections in Tehran, Iran. Gene Rep 24:101267",
"volume": "24",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2022.919402",
"author": "F Azizi Jalilian",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "1690_CR4",
"unstructured": "Azizi Jalilian F, Keshavarz G, Khazaei S, Nezamdoost M, Hashemi SH, Mamani M, Ansari N, Amini R, Khalkhali A, Keshavarz A (2022) The effects of nutrition bio-shield superfood powder on immune system function: a clinical trial study among patients with COVID-19. Front Immunol 13:919402",
"volume": "13",
"year": "2022"
},
{
"author": "A Bayat",
"first-page": "6",
"issue": "1",
"journal-title": "Int J Nutr Food Eng",
"key": "1690_CR5",
"unstructured": "Bayat A, Khalkhali A, Mahjoub AR (2014) Nutrition bio-shield superfood: healthy and live herbal supplement for immune system enhancement. Int J Nutr Food Eng 15(1):6–9",
"volume": "15",
"year": "2014"
},
{
"DOI": "10.1186/s13063-020-04547-0",
"author": "MT Beigmohammadi",
"doi-asserted-by": "publisher",
"first-page": "614",
"issue": "1",
"journal-title": "Trials",
"key": "1690_CR6",
"unstructured": "Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, M. Mahmoodi Ali Abadi and M. Foroumandi, (2020) Impact of vitamins A, B, C, D, and E supplementation on improvement and mortality rate in ICU patients with coronavirus-19: a structured summary of a study protocol for a randomized controlled trial. Trials 21(1):614",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1186/s13063-021-05795-4",
"author": "MT Beigmohammadi",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Trials",
"key": "1690_CR7",
"unstructured": "Beigmohammadi MT, Bitarafan S, Hoseindokht A, Abdollahi A, Amoozadeh L, Soltani D (2021) The effect of supplementation with vitamins A, B, C, D, and E on disease severity and inflammatory responses in patients with COVID-19: a randomized clinical trial. Trials 22(1):1–9",
"volume": "22",
"year": "2021"
},
{
"DOI": "10.1186/s13063-020-04819-9",
"author": "A Emadi",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "1",
"journal-title": "Trials",
"key": "1690_CR8",
"unstructured": "Emadi A, Chua JV, Talwani R, Bentzen SM, Baddley J (2020) Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: a structured summary of a study protocol for a randomised controlled trial. Trials 21(1):1–5",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1038/s41392-022-00997-x",
"author": "Y Fan",
"doi-asserted-by": "publisher",
"first-page": "141",
"issue": "1",
"journal-title": "Signal Transduct Target Ther",
"key": "1690_CR9",
"unstructured": "Fan Y, Li X, Zhang L, Wan S, Zhang L, Zhou F (2022) SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal Transduct Target Ther 7(1):141",
"volume": "7",
"year": "2022"
},
{
"DOI": "10.1016/j.virusres.2020.198171",
"author": "AC Gadotti",
"doi-asserted-by": "publisher",
"journal-title": "Virus Res",
"key": "1690_CR10",
"unstructured": "Gadotti AC, de Castro Deus M, Telles JP, Wind R, Goes M, Ossoski RGC, de Padua AM, de Noronha L, Moreno-Amaral A, Baena CP (2020) IFN-γ is an independent risk factor associated with mortality in patients with moderate and severe COVID-19 infection. Virus Res 289:198171",
"volume": "289",
"year": "2020"
},
{
"DOI": "10.3390/nu13114047",
"author": "MS Gönen",
"doi-asserted-by": "publisher",
"first-page": "4047",
"issue": "11",
"journal-title": "Nutrients",
"key": "1690_CR11",
"unstructured": "Gönen MS, Alaylıoğlu M, Durcan E, Özdemir Y, Şahin S, Konukoğlu D, Nohut OK, Ürkmez S, Küçükece B, Balkan İİ (2021) Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients 13(11):4047",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.1111/sji.12967",
"author": "A Jafarzadeh",
"doi-asserted-by": "publisher",
"issue": "2",
"journal-title": "Scand J Immunol",
"key": "1690_CR12",
"unstructured": "Jafarzadeh A, Jafarzadeh S, Nozari P, Mokhtari P, Nemati M (2021) Lymphopenia an important immunological abnormality in patients with COVID-19: possible mechanisms. Scand J Immunol 93(2):e12967",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1002/jmv.27515",
"author": "M Kandeel",
"doi-asserted-by": "publisher",
"first-page": "1627",
"issue": "4",
"journal-title": "J Med Virol",
"key": "1690_CR13",
"unstructured": "Kandeel M, Mohamed ME, Abd El-Lateef HM, Venugopala KN, El-Beltagi HS (2022) Omicron variant genome evolution and phylogenetics. J Med Virol 94(4):1627–1632",
"volume": "94",
"year": "2022"
},
{
"DOI": "10.1016/j.freeradbiomed.2010.02.011",
"author": "H Kuwahara",
"doi-asserted-by": "publisher",
"first-page": "1252",
"issue": "9",
"journal-title": "Free Radical Biol Med",
"key": "1690_CR14",
"unstructured": "Kuwahara H, Horie T, Ishikawa S, Tsuda C, Kawakami S, Noda Y, Kaneko T, Tahara S, Tachibana T, Okabe M (2010) Oxidative stress in skeletal muscle causes severe disturbance of exercise activity without muscle atrophy. Free Radical Biol Med 48(9):1252–1262",
"volume": "48",
"year": "2010"
},
{
"DOI": "10.1038/d41573-020-00073-5",
"author": "TT Le",
"doi-asserted-by": "publisher",
"first-page": "305",
"issue": "5",
"journal-title": "Nat Rev Drug Discov",
"key": "1690_CR15",
"unstructured": "Le TT, Andreadakis Z, Kumar A, Román RG, Tollefsen S, Saville M, Mayhew S (2020) The COVID-19 vaccine development landscape. Nat Rev Drug Discov 19(5):305–306",
"volume": "19",
"year": "2020"
},
{
"DOI": "10.1136/bmjopen-2021-057024",
"author": "M Legacy",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "BMJ Open",
"key": "1690_CR16",
"unstructured": "Legacy M, Seely D, Conte E, Psihogios A, Ramsay T, Fergusson DA, Kanji S, Simmons J-G, Wilson K (2022) Dietary supplements to reduce symptom severity and duration in people with SARS-CoV-2: study protocol for a randomised, double-blind, placebo controlled clinical trial. BMJ Open 12(3):e057024",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.1371/journal.pone.0246087",
"author": "TW Leulseged",
"doi-asserted-by": "publisher",
"issue": "3",
"journal-title": "PLoS ONE",
"key": "1690_CR17",
"unstructured": "Leulseged TW, Hassen IS, Ayele BT, Tsegay YG, Abebe DS, Edo MG, Maru EH, Zewde WC, Naylor LK, Semane DF (2021) Laboratory biomarkers of COVID-19 disease severity and outcome: findings from a developing country. PLoS ONE 16(3):e0246087",
"volume": "16",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(20)30628-0",
"author": "P Mehta",
"doi-asserted-by": "publisher",
"first-page": "1033",
"issue": "10229",
"journal-title": "The Lancet",
"key": "1690_CR18",
"unstructured": "Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 395(10229):1033–1034",
"volume": "395",
"year": "2020"
},
{
"author": "S Meo",
"first-page": "8012",
"issue": "24",
"journal-title": "Eur Rev Med Pharmacol Sci",
"key": "1690_CR19",
"unstructured": "Meo S, Meo A, Al-Jassir F, Klonoff D (2021) Omicron SARS-CoV-2 new variant: global prevalence and biological and clinical characteristics. Eur Rev Med Pharmacol Sci 25(24):8012–8018",
"volume": "25",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2021.592727",
"author": "E Mortaz",
"doi-asserted-by": "publisher",
"journal-title": "Front Immunol",
"key": "1690_CR20",
"unstructured": "Mortaz E, Tabarsi P, Jamaati H, Dalil Roofchayee N, Dezfuli NK, Hashemian SM, Moniri A, Marjani M, Malekmohammad M, Mansouri D (2021) Increased serum levels of soluble TNF-α receptor is associated with ICU mortality in COVID-19 patients. Front Immunol 12:592727",
"volume": "12",
"year": "2021"
},
{
"DOI": "10.1016/j.micpath.2022.105792",
"author": "M Mosadegh",
"doi-asserted-by": "publisher",
"journal-title": "Microb Pathog",
"key": "1690_CR21",
"unstructured": "Mosadegh M, Khalkhali A, Erfani Y, Nezamdoost M (2022a) The effect of Nutrition Bio-shield superfood (NBS) on disease severity and laboratory biomarkers in patients with COVID-19: a randomized clinical trial. Microb Pathog 172:105792",
"volume": "172",
"year": "2022"
},
{
"DOI": "10.4274/tji.galenos.2022.03522",
"author": "M Mosadegh",
"doi-asserted-by": "publisher",
"first-page": "95",
"issue": "2",
"journal-title": "Turk J Immunol",
"key": "1690_CR22",
"unstructured": "Mosadegh M, Khalkhali A, Sadeghi Y, Erfani Y (2022b) The anti-inflammatory effects of the nutrition bio-shield (NBS) supplement intake on adjuvant-induced rheumatoid arthritis in rat. Turk J Immunol 10(2):95–101",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1007/s40618-021-01639-9",
"author": "J Oristrell",
"doi-asserted-by": "publisher",
"first-page": "167",
"journal-title": "J Endocrinol Invest",
"key": "1690_CR23",
"unstructured": "Oristrell J, Oliva JC, Casado E, Subirana I, Domínguez D, Toloba A, Balado A, Grau M (2022) Vitamin D supplementation and COVID-19 risk: a population-based, cohort study. J Endocrinol Invest 45:167–179",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"author": "D Planas",
"doi-asserted-by": "publisher",
"first-page": "276",
"issue": "7871",
"journal-title": "Nature",
"key": "1690_CR24",
"unstructured": "Planas D, Veyer D, Baidaliuk A, Staropoli I, Guivel-Benhassine F, Rajah MM, Planchais C, Porrot F, Robillard N, Puech J (2021) Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596(7871):276–280",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.1007/s00134-020-05991-x",
"author": "Q Ruan",
"doi-asserted-by": "publisher",
"first-page": "846",
"issue": "5",
"journal-title": "Intensive Care Med",
"key": "1690_CR25",
"unstructured": "Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 46(5):846–848",
"volume": "46",
"year": "2020"
},
{
"author": "TD Shanafelt",
"first-page": "4188",
"issue": "18",
"journal-title": "Blood J Am Soc Hematol",
"key": "1690_CR26",
"unstructured": "Shanafelt TD, Kay NE, Jenkins G, Call TG, Zent CS, Jelinek DF, Morice WG, Boysen J, Zakko L, Schwager S (2009) B-cell count and survival: differentiating chronic lymphocytic leukemia from monoclonal B-cell lymphocytosis based on clinical outcome. Blood J Am Soc Hematol 113(18):4188–4196",
"volume": "113",
"year": "2009"
},
{
"author": "LL Speakman",
"first-page": "2",
"issue": "2021",
"journal-title": "Drugs in Context",
"key": "1690_CR27",
"unstructured": "Speakman LL, Michienzi SM, Badowski ME (2021) Vitamins, supplements and COVID-19: a review of currently available evidence. Drugs in Context 10(2021):2–6",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.3389/fimmu.2018.00754",
"author": "NR Sproston",
"doi-asserted-by": "publisher",
"first-page": "754",
"journal-title": "Front Immunol",
"key": "1690_CR28",
"unstructured": "Sproston NR, Ashworth JJ (2018) Role of C-reactive protein at sites of inflammation and infection. Front Immunol 9:754",
"volume": "9",
"year": "2018"
},
{
"DOI": "10.1038/s41586-021-03402-9",
"author": "H Tegally",
"doi-asserted-by": "publisher",
"first-page": "438",
"issue": "7854",
"journal-title": "Nature",
"key": "1690_CR29",
"unstructured": "Tegally H, Wilkinson E, Giovanetti M, Iranzadeh A, Fonseca V, Giandhari J, Doolabh D, Pillay S, San EJ, Msomi N (2021) Detection of a SARS-CoV-2 variant of concern in South Africa. Nature 592(7854):438–443",
"volume": "592",
"year": "2021"
},
{
"DOI": "10.58676/sjmas.v1i2.11",
"author": "N Tzenios",
"doi-asserted-by": "publisher",
"journal-title": "Special J Med Acad Other Life Sci",
"key": "1690_CR30",
"unstructured": "Tzenios N, Chahine M, Tazanios M (2023) Better strategies for coronavirus (COVID-19) vaccination. Special J Med Acad Other Life Sci. https://doi.org/10.58676/sjmas.v1i2.11",
"year": "2023"
},
{
"DOI": "10.1038/s41586-021-03398-2",
"author": "P Wang",
"doi-asserted-by": "publisher",
"first-page": "130",
"issue": "7857",
"journal-title": "Nature",
"key": "1690_CR31",
"unstructured": "Wang P, Nair MS, Liu L, Iketani S, Luo Y, Guo Y, Wang M, Yu J, Zhang B, Kwong PD (2021) Antibody resistance of SARS-CoV-2 variants B. 1.351 and B. 1.1. 7. Nature 593(7857):130–135",
"volume": "593",
"year": "2021"
},
{
"DOI": "10.1093/ajcp/aqaa205",
"author": "MD Weidmann",
"doi-asserted-by": "publisher",
"first-page": "333",
"issue": "3",
"journal-title": "Am J Clin Pathol",
"key": "1690_CR32",
"unstructured": "Weidmann MD, Ofori K, Rai AJ (2021) Laboratory biomarkers in the management of patients with COVID-19. Am J Clin Pathol 155(3):333–342",
"volume": "155",
"year": "2021"
},
{
"author": "Y Yao",
"first-page": "1",
"issue": "49",
"journal-title": "J Intensive Care",
"key": "1690_CR33",
"unstructured": "Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z, Chen X, Chen S, Yu K, Huang Z (2020) D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: a case control study. J Intensive Care 8(49):1–11",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"author": "F Zhou",
"doi-asserted-by": "publisher",
"first-page": "1054",
"issue": "10229",
"journal-title": "Lancet",
"key": "1690_CR34",
"unstructured": "Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062",
"volume": "395",
"year": "2020"
}
],
"reference-count": 34,
"references-count": 34,
"relation": {},
"resource": {
"primary": {
"URL": "https://amb-express.springeropen.com/articles/10.1186/s13568-024-01690-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Applied Microbiology and Biotechnology",
"Biophysics"
],
"subtitle": [],
"title": "NBS superfood: a promising adjunctive therapy in critically ill ICU patients with omicron variant of COVID-19",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "14"
}
mosadegh2