Tenofovir Disoproxil Fumarate/Emtricitabine and Baricitinib for Patients at High Risk of Severe Coronavirus Disease 2019: The PANCOVID Randomized Clinical Trial
et al., Clinical Infectious Diseases, doi:10.1093/cid/ciac628, PANCOVID, Aug 2022
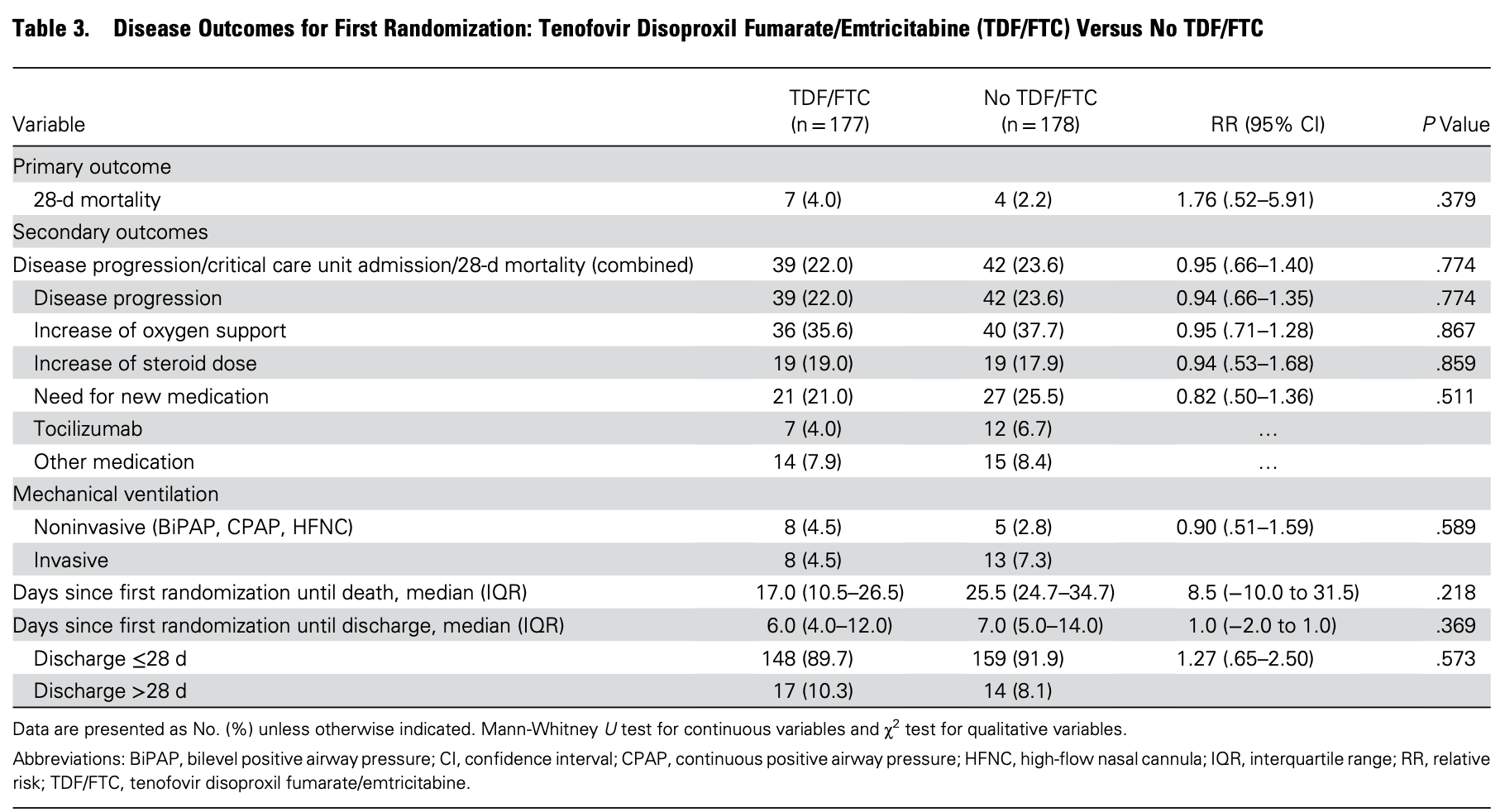
RCT 355 high-risk, mostly hospitalized COVID-19 patients showing no significant benefit with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) treatment.
|
risk of death, 76.0% higher, RR 1.76, p = 0.38, treatment 177, control 178.
|
|
risk of progression, 6.0% lower, RR 0.94, p = 0.77, treatment 177, control 178.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Montejano et al., 20 Aug 2022, Randomized Controlled Trial, Spain, peer-reviewed, median age 67.0, 32 authors, study period 10 October, 2020 - 23 September, 2021, PANCOVID trial.
Contact: arribas@salud.madrid.org.
Tenofovir Disoproxil Fumarate/Emtricitabine and Baricitinib for Patients at High Risk of Severe Coronavirus Disease 2019: The PANCOVID Randomized Clinical Trial
Clinical Infectious Diseases, doi:10.1093/cid/ciac628
Background. This study was designed to evaluate if patients with high risk for severe coronavirus disease 2019 would benefit from treatment with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) followed by baricitinib in case of hypoxemia and systemic inflammation.
Methods. PANCOVID is an open-label, double-randomized, phase 3 pragmatic clinical trial including adults with symptomatic COVID-19 with ≥2 comorbidities or aged ≥60 years and was conducted between 10 October 2020 and 23 September 2021. In the first randomization, patients received TDF/FTC or no TDF/FTC. In the second randomization, patients with room air oxygen saturation <95% and at least 1 increased inflammatory biomarker received baricitinib plus dexamethasone or dexamethasone alone. The primary endpoint was 28-day mortality. Main secondary endpoint was 28-day disease progression or critical care unit admission or mortality. The trial was stopped before reaching planned sample size due to the decrease in the number of cases and a mortality rate substantially lower than expected. Results. Of the 355 included participants, 97% were hospitalized at baseline. Overall, 28-day mortality was 3.1%. The 28-day mortality relative risk (RR) for participants treated with TDF/FTC was 1.76 (95% confidence interval [CI], .52-5.91; P = .379); it was 0.42 (95% CI, .11-1.59; P = .201) for those treated with baricitinib. The 28-day RR for the main secondary combined endpoint for participants treated with TDF/FTC was 0.95 (95% CI, .66-1.40; P = .774); it was 0.90 (95% CI, .61-1.33; P = .687) for those treated with baricitinib. Conclusions. Our results do not suggest a beneficial effect of TDF/FTC; nevertheless, they are compatible with the beneficial effect of baricitinib already established by other clinical trials.
Supplementary Data Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author. Financial support. This clinical trial was funded by the Instituto de Salud Carlos III (ISCIII), Ministry of Innovation and Science of Spain, in a competitive and public grant (Royal Decree-Law 8/2020, of 17 March, on extraordinary urgent measures to face the economic and social impact of COVID-19). Project code: COV20/00023 (co-funded by European Regional Development Fund/European Social Fund "A way to make Europe"/"Investing in your future"). Baricitinib was provided by Eli Lilly, and tenofovir disoproxil fumarate/emtricitabine was partially provided by Teva. The clinical trial was designed and the data analyzed by the senior authors and the biostatistician. Potential conflicts of interest. P. R. has received grant support and honoraria from Gilead and MSD; consulting fees from AbbVie SL; payment or honoraria for lectures, presentations, speaker's bureaus, manuscript writing, or educational events from ViiV and Gilead Sciences; and support for attending meetings and/or travel from AbbVie and GSK (ViiV). J. V. has received scholarships and honorarium as speaker for Gilead Sciences. M. S. has received honoraria from Gilead; has developed..
References
Agarwal, Rochwerg, Lamontagne, A living WHO guideline on drugs for covid-19, BMJ
Amo, Polo, Moreno, Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study, Ann Intern Med
Arribas, Bhagani, Lobo, Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19, NEJM Evidence
Berenguer, Díez, Martín-Vicente, Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort, Clin Microbiol Infect
Bernal, Da Silva, Musungaie, Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients, N Engl J Med
Borobia, Carcas, Arnalich, A cohort of patients with COVID-19 in a major teaching hospital in Europe, J Clin Med
Castillo-Mancilla, Meditz, Wilson, Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals, J Acquir Immune Defic Syndr
Choy, Wong, Kaewpreedee, Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro, Antiviral Res
Clososki, Soldi, Da Silva, Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2, J Braz Chem Soc
Dal-Ré, Janiaud, Ioannidis, Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic?, BMC Med
Elfiky, Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study, Life Sci
Ely, Ramanan, Kartman, Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial, Lancet Respir Med
Feng, Bilello, Babusis, NRTIs tenofovir, TAF, TDF, and FTC are inactive against SARS-CoV-2
Gaitán-Duarte, Álvarez-Moreno, Cj, Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial, EClinicalMedicine
Group, Horby, Emberson, Mafham, Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis, Lancet
Kalil, Patterson, Mehta, Baricitinib plus remdesivir for hospitalized adults with Covid-19, N Engl J Med
Marconi, Ramanan, De Bono, Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial, Lancet Respir Med
Melchjorsen, Risør, Søgaard, Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells, J Acquir Immune Defic Syndr
Munoz, Buti, Vazquez, Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients, J Hepatol
Parienti, Prazuck, Peyro-Saint-Paul, Effect of tenofovir disoproxil fumarate and emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: a pilot, randomized, open-label phase 2 trial, EClinicalMedicine
Park, Yu, Kim, Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets, mBio
Selvaraj, Finn, Lal, Khan, Dapaah-Afriyie et al., Baricitinib in hospitalised patients with COVID-19: a meta-analysis of randomised controlled trials, EClinicalMedicine
Sims, Krishnan, Chang, Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19, J Allergy Clin Immunol
Stebbing, Phelan, Griffin, COVID-19: combining antiviral and antiinflammatory treatments, Lancet Infect Dis
Tay, Poh, Rénia, Macary, Ng, The trinity of COVID-19: immunity, inflammation and intervention, Nat Rev Immunol
DOI record:
{
"DOI": "10.1093/cid/ciac628",
"ISSN": [
"1058-4838",
"1537-6591"
],
"URL": "http://dx.doi.org/10.1093/cid/ciac628",
"abstract": "<jats:title>Abstract</jats:title>\n <jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>This study was designed to evaluate if patients with high risk for severe coronavirus disease 2019 (COVID-19) would benefit from treatment with tenofovir disoproxil fumarate/emtricitabine (TDF/FTC) followed by baricitinib in case of hypoxemia and systemic inflammation.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>PANCOVID is an open-label, double-randomized, phase 3 pragmatic clinical trial including adults with symptomatic COVID-19 with ≥2 comorbidities or aged ≥60 years and was conducted between 10 October 2020 and 23 September 2021. In the first randomization, patients received TDF/FTC or no TDF/FTC. In the second randomization, patients with room air oxygen saturation &lt;95% and at least 1 increased inflammatory biomarker received baricitinib plus dexamethasone or dexamethasone alone. The primary endpoint was 28-day mortality. Main secondary endpoint was 28-day disease progression or critical care unit admission or mortality. The trial was stopped before reaching planned sample size due to the decrease in the number of cases and a mortality rate substantially lower than expected.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of the 355 included participants, 97% were hospitalized at baseline. Overall, 28-day mortality was 3.1%. The 28-day mortality relative risk (RR) for participants treated with TDF/FTC was 1.76 (95% confidence interval [CI], .52–5.91; P = .379); it was 0.42 (95% CI, .11–1.59; P = .201) for those treated with baricitinib. The 28-day RR for the main secondary combined endpoint for participants treated with TDF/FTC was 0.95 (95% CI, .66–1.40; P = .774); it was 0.90 (95% CI, .61–1.33; P = .687) for those treated with baricitinib.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Our results do not suggest a beneficial effect of TDF/FTC; nevertheless, they are compatible with the beneficial effect of baricitinib already established by other clinical trials.</jats:p>\n </jats:sec>\n <jats:sec>\n <jats:title>Clinical Trials Registration</jats:title>\n <jats:p>EudraCT: 2020-001156-18.</jats:p>\n </jats:sec>",
"author": [
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Internal Medicine Department, La Paz University Hospital, Instituto de Investigación del Hospital Universitario La Paz, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas , Madrid , Spain"
}
],
"family": "Montejano",
"given": "Rocío",
"sequence": "first"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Internal Medicine Department, La Paz University Hospital, Instituto de Investigación del Hospital Universitario La Paz, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas , Madrid , Spain"
}
],
"family": "de la Calle-Prieto",
"given": "Fernando",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases, Research Unit, University Hospital Fundación Alcorcón , Madrid , Spain"
}
],
"family": "Velasco",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Unit, University Hospital Fundación Alcorcón, Rey Juan Carlos University , Madrid , Spain"
}
],
"family": "Guijarro",
"given": "Carlos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacology Department, La Paz University Hospital, Infectious Diseases Unit, La Paz University Hospital, Instituto de Investigación del Hospital Universitario La Paz , Madrid , Spain"
}
],
"family": "Queiruga-Parada",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacology Department, La Paz University Hospital, La Paz University Hospital, Instituto de Investigación del Hospital Universitario La Paz, Spanish Clinical Research Network , Madrid , Spain"
}
],
"family": "Jiménez-González",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, University Hospital Infanta Sofía , Madrid , Spain"
}
],
"family": "González-Ruano",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, University Hospital Infanta Sofía , Madrid , Spain"
}
],
"family": "Martínez",
"given": "Patricia",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Cruces University Hospital , Barakaldo , Spain"
}
],
"family": "Goikoetxea",
"given": "Ane Josune",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Cruces University Hospital , Barakaldo , Spain"
}
],
"family": "Ibarrola",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Internal Medicine Department, La Princesa University Hospital , Madrid , Spain"
}
],
"family": "Ciudad",
"given": "Marianela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Internal Medicine Department, La Princesa University Hospital , Madrid , Spain"
}
],
"family": "Gutiérrez",
"given": "Ángela",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Guadalajara University Hospital, University of Alcalá , Spain"
}
],
"family": "Torralba",
"given": "Miguel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Guadalajara University Hospital, University of Alcalá , Spain"
}
],
"family": "Díaz-Brasero",
"given": "Ana",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-4212-7419",
"affiliation": [
{
"name": "Infanta Leonor University Hospital, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, School of Medicine, Complutense University , Madrid , Spain"
}
],
"authenticated-orcid": false,
"family": "Ryan",
"given": "Pablo",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-6187-7442",
"affiliation": [
{
"name": "Hospital de Emergencias Enfermera Isabel Zendal , Madrid , Spain"
}
],
"authenticated-orcid": false,
"family": "Marcelo",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Microbiology and Infectious Diseases Department, Gregorio Marañón University Hospital, Instituto de Investigación Sanitaria Gregorio Marañón , Madrid , Spain"
}
],
"family": "Díez",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department, Basurto University Hospital , Basurto , Spain"
}
],
"family": "Ibarra",
"given": "Sofía",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Alicante General University Hospital, Alicante Institute of Health and Biomedical Research , Alicante , Spain"
}
],
"family": "Merino",
"given": "Esperanza",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Internal Medicine Department, Clínico San Carlos University Hospital, El Instituto de Investigación Sanitaria del Hospital Clínico San Carlos, Madrid , Spain"
}
],
"family": "Estrada",
"given": "Vicente",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, University Hospital Fundación Alcorcón , Madrid , Spain"
}
],
"family": "Marcos",
"given": "Javier",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, Príncipe de Asturias University Hospital , Alcalá de Henares , Spain"
}
],
"family": "Novella",
"given": "María",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Emergency Department, La Paz University Hospital , Madrid , Spain"
}
],
"family": "Rivera",
"given": "María A",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, University Hospital Fundación Alcorcón , Madrid , Spain"
}
],
"family": "Ruiz-Muñoz",
"given": "Manuel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Fundación SEIMC-GESIDA , Madrid , Spain"
}
],
"family": "de Miguel",
"given": "Marta",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Internal Medicine Department, University Hospital Infanta Sofía , Madrid , Spain"
}
],
"family": "Soler",
"given": "Llanos",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Unit, Cruces University Hospital , Barakaldo , Spain"
}
],
"family": "del Álamo",
"given": "Mikel",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Infectious Diseases Department, Ramón y Cajal University Hospital, Instituto Ramón y Cajal de Investigación Sanitaria, University of Alcalá School of Medicine, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas , Madrid , Spain"
}
],
"family": "Moreno",
"given": "Santiago",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacology Department, La Paz University Hospital, Instituto de Investigación del Hospital Universitario La Paz, School of Medicine, Autonomous University of Madrid, Spanish Clinical Research Network, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas , Madrid , Spain"
}
],
"family": "Carcas",
"given": "Antonio J",
"sequence": "additional"
},
{
"affiliation": [
{
"name": "Clinical Pharmacology Department, La Paz University Hospital, Instituto de Investigación del Hospital Universitario La Paz, School of Medicine, Autonomous University of Madrid, Spanish Clinical Research Network, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas , Madrid , Spain"
}
],
"family": "Borobia",
"given": "Alberto M",
"sequence": "additional"
},
{
"ORCID": "https://orcid.org/0000-0002-7410-9450",
"affiliation": [
{
"name": "Infectious Diseases Unit, Internal Medicine Department, La Paz University Hospital, Instituto de Investigación del Hospital Universitario La Paz, School of Medicine, Autonomous University of Madrid, Centro de Investigación Biomédica en Red de Enfermedades Infecciosas , Madrid , Spain"
}
],
"authenticated-orcid": false,
"family": "Arribas",
"given": "José R",
"sequence": "additional"
},
{
"affiliation": [],
"name": "for the PANCOVID Study Group",
"sequence": "additional"
}
],
"container-title": "Clinical Infectious Diseases",
"content-domain": {
"crossmark-restriction": false,
"domain": []
},
"created": {
"date-parts": [
[
2022,
7,
30
]
],
"date-time": "2022-07-30T08:19:38Z",
"timestamp": 1659169178000
},
"deposited": {
"date-parts": [
[
2023,
2,
8
]
],
"date-time": "2023-02-08T17:47:40Z",
"timestamp": 1675878460000
},
"funder": [
{
"DOI": "10.13039/501100004587",
"doi-asserted-by": "crossref",
"id": [
{
"asserted-by": "crossref",
"id": "10.13039/501100004587",
"id-type": "DOI"
}
],
"name": "Instituto de Salud Carlos III"
},
{
"name": "Ministry of Innovation and Science of Spain,"
}
],
"indexed": {
"date-parts": [
[
2025,
6,
8
]
],
"date-time": "2025-06-08T19:24:23Z",
"timestamp": 1749410663955,
"version": "3.37.3"
},
"is-referenced-by-count": 15,
"issue": "3",
"issued": {
"date-parts": [
[
2022,
8,
20
]
]
},
"journal-issue": {
"issue": "3",
"published-online": {
"date-parts": [
[
2022,
8,
20
]
]
},
"published-print": {
"date-parts": [
[
2023,
2,
8
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://academic.oup.com/pages/standard-publication-reuse-rights",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
8,
20
]
],
"date-time": "2022-08-20T00:00:00Z",
"timestamp": 1660953600000
}
}
],
"link": [
{
"URL": "https://academic.oup.com/cid/advance-article-pdf/doi/10.1093/cid/ciac628/45485594/ciac628.pdf",
"content-type": "application/pdf",
"content-version": "am",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/76/3/e116/49125319/ciac628.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "syndication"
},
{
"URL": "https://academic.oup.com/cid/article-pdf/76/3/e116/49125319/ciac628.pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "286",
"original-title": [],
"page": "e116-e125",
"prefix": "10.1093",
"published": {
"date-parts": [
[
2022,
8,
20
]
]
},
"published-online": {
"date-parts": [
[
2022,
8,
20
]
]
},
"published-other": {
"date-parts": [
[
2023,
2,
1
]
]
},
"published-print": {
"date-parts": [
[
2023,
2,
8
]
]
},
"publisher": "Oxford University Press (OUP)",
"reference": [
{
"DOI": "10.1016/j.lfs.2020.117592",
"article-title": "Ribavirin, remdesivir, sofosbuvir, galidesivir, and tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study",
"author": "Elfiky",
"doi-asserted-by": "crossref",
"journal-title": "Life Sci",
"key": "2023020817090831300_ciac628-B1",
"volume": "253",
"year": "2020"
},
{
"article-title": "Tenofovir disoproxil fumarate: new chemical developments and encouraging in vitro biological results for SARS-CoV-2",
"author": "Clososki",
"first-page": "1552",
"journal-title": "J Braz Chem Soc",
"key": "2023020817090831300_ciac628-B2",
"volume": "31",
"year": "2020"
},
{
"DOI": "10.1016/j.antiviral.2020.104786",
"article-title": "Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro",
"author": "Choy",
"doi-asserted-by": "crossref",
"journal-title": "Antiviral Res",
"key": "2023020817090831300_ciac628-B3",
"volume": "178",
"year": "2020"
},
{
"author": "Feng",
"key": "2023020817090831300_ciac628-B4",
"year": "2021"
},
{
"DOI": "10.1128/mBio.01114-20",
"article-title": "Antiviral efficacies of FDA-approved drugs against SARS-CoV-2 infection in ferrets",
"author": "Park",
"doi-asserted-by": "crossref",
"journal-title": "mBio",
"key": "2023020817090831300_ciac628-B5",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1016/j.cmi.2021.06.023",
"article-title": "Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort",
"author": "Berenguer",
"doi-asserted-by": "crossref",
"first-page": "1678",
"journal-title": "Clin Microbiol Infect",
"key": "2023020817090831300_ciac628-B6",
"volume": "27",
"year": "2021"
},
{
"DOI": "10.7326/M20-3689",
"article-title": "Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study",
"author": "Del Amo",
"doi-asserted-by": "crossref",
"first-page": "536",
"journal-title": "Ann Intern Med",
"key": "2023020817090831300_ciac628-B7",
"volume": "173",
"year": "2020"
},
{
"article-title": "Tenofovir reduces the severity of COVID-19 infection in chronic hepatitis B patients",
"author": "Munoz",
"first-page": "S746",
"journal-title": "J Hepatol",
"key": "2023020817090831300_ciac628-B8",
"volume": "75",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.100993",
"article-title": "Effect of tenofovir disoproxil fumarate and emtricitabine on nasopharyngeal SARS-CoV-2 viral load burden amongst outpatients with COVID-19: a pilot, randomized, open-label phase 2 trial",
"author": "Parienti",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "2023020817090831300_ciac628-B9",
"volume": "38",
"year": "2021"
},
{
"DOI": "10.1016/j.eclinm.2021.101242",
"article-title": "Effectiveness of rosuvastatin plus colchicine, emtricitabine/tenofovir and combinations thereof in hospitalized patients with COVID-19: a pragmatic, open-label randomized trial",
"author": "Gaitán-Duarte",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "2023020817090831300_ciac628-B10",
"volume": "43",
"year": "2022"
},
{
"DOI": "10.1097/QAI.0000000000000529",
"article-title": "Reduced immune activation during tenofovir-emtricitabine therapy in HIV-negative individuals",
"author": "Castillo-Mancilla",
"doi-asserted-by": "crossref",
"first-page": "495",
"journal-title": "J Acquir Immune Defic Syndr",
"key": "2023020817090831300_ciac628-B11",
"volume": "68",
"year": "2015"
},
{
"DOI": "10.1097/QAI.0b013e3182185276",
"article-title": "Tenofovir selectively regulates production of inflammatory cytokines and shifts the IL-12/IL-10 balance in human primary cells",
"author": "Melchjorsen",
"doi-asserted-by": "crossref",
"first-page": "265",
"journal-title": "J Acquir Immune Defic Syndr",
"key": "2023020817090831300_ciac628-B12",
"volume": "57",
"year": "2011"
},
{
"DOI": "10.1038/s41577-020-0311-8",
"article-title": "The trinity of COVID-19: immunity, inflammation and intervention",
"author": "Tay",
"doi-asserted-by": "crossref",
"first-page": "363",
"journal-title": "Nat Rev Immunol",
"key": "2023020817090831300_ciac628-B13",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/S2213-2600(21)00331-3",
"article-title": "Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial",
"author": "Marconi",
"doi-asserted-by": "crossref",
"first-page": "1407",
"journal-title": "Lancet Respir Med",
"key": "2023020817090831300_ciac628-B14",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031994",
"article-title": "Baricitinib plus remdesivir for hospitalized adults with Covid-19",
"author": "Kalil",
"doi-asserted-by": "crossref",
"first-page": "795",
"journal-title": "N Engl J Med",
"key": "2023020817090831300_ciac628-B15",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/S0140-6736(22)01109-6",
"article-title": "Baricitinib in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial and updated meta-analysis",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "crossref",
"first-page": "359",
"journal-title": "Lancet",
"key": "2023020817090831300_ciac628-B16",
"volume": "400",
"year": "2022"
},
{
"DOI": "10.1016/S1473-3099(20)30132-8",
"article-title": "COVID-19: combining antiviral and anti-inflammatory treatments",
"author": "Stebbing",
"doi-asserted-by": "crossref",
"first-page": "400",
"journal-title": "Lancet Infect Dis",
"key": "2023020817090831300_ciac628-B17",
"volume": "20",
"year": "2020"
},
{
"DOI": "10.1016/j.jaci.2020.08.031",
"article-title": "Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19",
"author": "Sims",
"doi-asserted-by": "crossref",
"first-page": "107",
"journal-title": "J Allergy Clin Immunol",
"key": "2023020817090831300_ciac628-B18",
"volume": "147",
"year": "2021"
},
{
"article-title": "A living WHO guideline on drugs for covid-19",
"author": "Agarwal",
"journal-title": "BMJ",
"key": "2023020817090831300_ciac628-B19",
"volume": "370",
"year": "2020"
},
{
"DOI": "10.3390/jcm9061733",
"article-title": "A cohort of patients with COVID-19 in a major teaching hospital in Europe",
"author": "Borobia",
"doi-asserted-by": "crossref",
"first-page": "E1733",
"journal-title": "J Clin Med",
"key": "2023020817090831300_ciac628-B20",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1016/j.eclinm.2022.101489",
"article-title": "Baricitinib in hospitalised patients with COVID-19: a meta-analysis of randomised controlled trials",
"author": "Selvaraj",
"doi-asserted-by": "crossref",
"journal-title": "EClinicalMedicine",
"key": "2023020817090831300_ciac628-B21",
"volume": "49",
"year": "2022"
},
{
"DOI": "10.1016/S2213-2600(22)00006-6",
"article-title": "Efficacy and safety of baricitinib plus standard of care for the treatment of critically ill hospitalised adults with COVID-19 on invasive mechanical ventilation or extracorporeal membrane oxygenation: an exploratory, randomised, placebo-controlled trial",
"author": "Ely",
"doi-asserted-by": "crossref",
"first-page": "327",
"journal-title": "Lancet Respir Med",
"key": "2023020817090831300_ciac628-B22",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1186/s12916-018-1038-2",
"article-title": "Real-world evidence: how pragmatic are randomized controlled trials labeled as pragmatic?",
"author": "Dal-Ré",
"doi-asserted-by": "crossref",
"first-page": "49",
"journal-title": "BMC Med",
"key": "2023020817090831300_ciac628-B23",
"volume": "16",
"year": "2018"
},
{
"DOI": "10.1056/NEJMoa2116044",
"article-title": "Molnupiravir for oral treatment of COVID-19 in nonhospitalized patients",
"author": "Jayk Bernal",
"doi-asserted-by": "crossref",
"first-page": "509",
"journal-title": "N Engl J Med",
"key": "2023020817090831300_ciac628-B24",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/EVIDoa2100044",
"article-title": "Randomized trial of molnupiravir or placebo in patients hospitalized with Covid-19",
"author": "Arribas",
"doi-asserted-by": "crossref",
"journal-title": "NEJM Evidence",
"key": "2023020817090831300_ciac628-B25",
"volume": "1",
"year": "2022"
}
],
"reference-count": 25,
"references-count": 25,
"relation": {},
"resource": {
"primary": {
"URL": "https://academic.oup.com/cid/article/76/3/e116/6652179"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Tenofovir Disoproxil Fumarate/Emtricitabine and Baricitinib for Patients at High Risk of Severe Coronavirus Disease 2019: The PANCOVID Randomized Clinical Trial",
"type": "journal-article",
"volume": "76"
}