Viral clearance and escape during therapy of COVID-19 outpatients: A prospective cohort study
et al., iScience, doi:10.1016/j.isci.2025.114226, Dec 2025
19th treatment shown to reduce risk in
March 2021, now with p = 0.000095 from 34 studies, recognized in 52 countries.
Efficacy is variant dependent.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
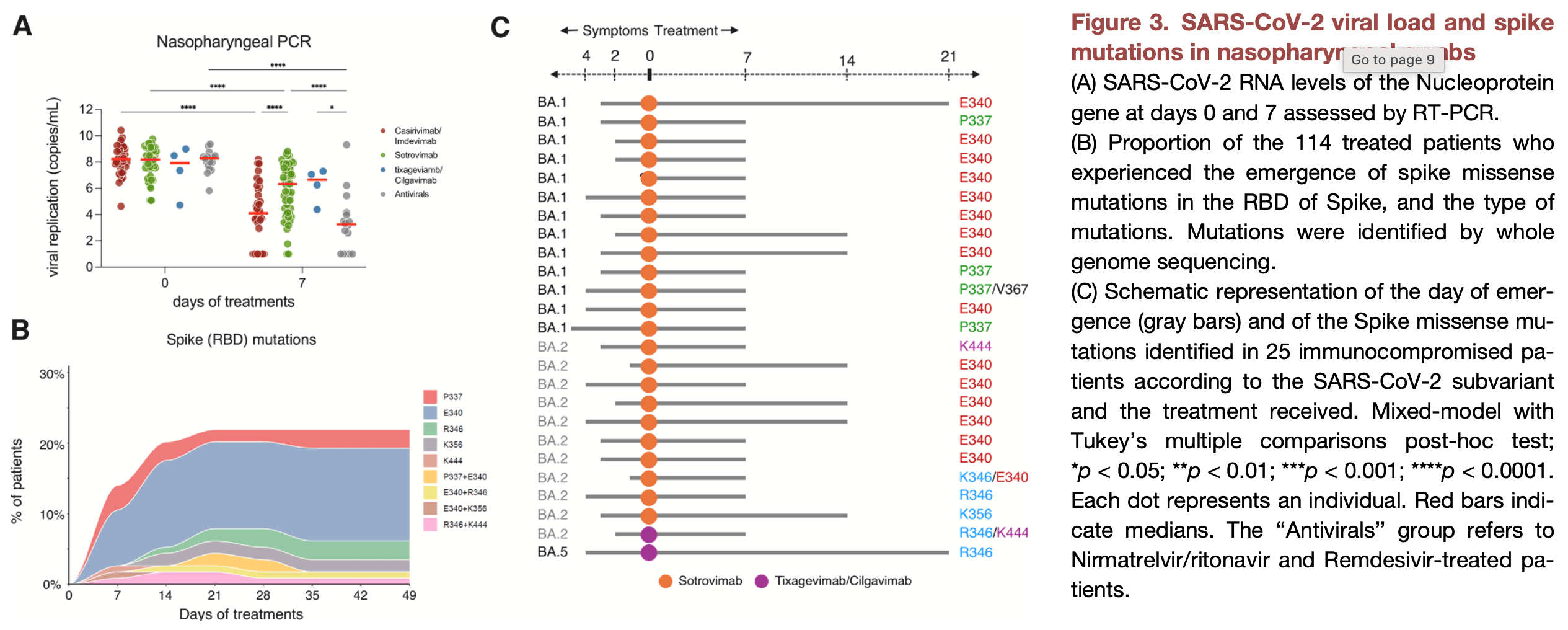
Prospective cohort study of 114 high-risk COVID-19 outpatients showing that monoclonal antibody treatments with suboptimal neutralizing activity against infecting variants resulted in higher viral loads and increased emergence of escape mutations.
Efficacy is variant dependent. In Vitro research suggests a lack of efficacy for many omicron variants1-7.
Study covers casirivimab/imdevimab, sotrovimab, and tixagevimab/cilgavimab.
1.
Liu et al., Striking Antibody Evasion Manifested by the Omicron Variant of SARS-CoV-2, bioRxiv, doi:10.1101/2021.12.14.472719.
2.
Sheward et al., Variable loss of antibody potency against SARS-CoV-2 B.1.1.529 (Omicron), bioRxiv, doi:10.1101/2021.12.19.473354.
3.
VanBlargan et al., An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by several therapeutic monoclonal antibodies, bioRxiv, doi:10.1101/2021.12.15.472828.
4.
Tatham et al., Lack of Ronapreve (REGN-CoV; casirivimab and imdevimab) virological efficacy against the SARS-CoV 2 Omicron variant (B.1.1.529) in K18-hACE2 mice, bioRxiv, doi:10.1101/2022.01.23.477397.
5.
Pochtovyi et al., In Vitro Efficacy of Antivirals and Monoclonal Antibodies against SARS-CoV-2 Omicron Lineages XBB.1.9.1, XBB.1.9.3, XBB.1.5, XBB.1.16, XBB.2.4, BQ.1.1.45, CH.1.1, and CL.1, Vaccines, doi:10.3390/vaccines11101533.
Martin-Blondel et al., 31 Dec 2025, prospective, France, peer-reviewed, median age 54.5, 24 authors, study period September 2021 - November 2022.
Contact: martin-blondel.g@chu-toulouse.fr (corresponding author), martin-blondel.g@chu-toulouse.fr (corresponding author), timothee.bruel@pasteur.fr.
Viral clearance and escape during therapy of COVID-19 outpatients: A prospective cohort study
iScience, doi:10.1016/j.isci.2025.114226
Baseline viral load, neutralization, and therapy govern viral clearance
RESOURCE AVAILABILITY Lead contact Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Timothe ´ e Bruel (timothee.bruel@pasteur.fr).
Materials availability All unique/stable reagents generated in this study are available from the corresponding authors with a completed Materials Transfer Agreement.
Data and code availability • This study did not generate any new codes. • The raw sequencing data contains traces of human genomic DNA and cannot be made publicly available according to French law. However, this data is available upon request. • The viral sequences are available on GISAID
STAR★METHODS Detailed methods are provided in the online version of this paper and include the following: Treatment was determined at the discretion of the treating physician. During the study period, patients received either a single infusion of Casirivimab/Imdevimab, 26 of Sotrovimab, 27 of Tixagevimab/Cilgavimab, 28 or 5 days of oral Nirmatrelvir/Ritonavir, 29 or 3 days of intravenous Remdesivir. 30 Baseline demographics, clinical and biological data were collected during the inclusion visit (day 0, prior to treatment initiation), then at day 7 and month 1. Nasopharyngeal (NP) swabs were collected at days 0 and 7, and repeated weekly in patients with a SARS-CoV-2 PCR with a cycle threshold (CT) < 31. Blood samples were obtained at days 0 and 7. The protocol has been approved by the ''CPP Sud-Est IV''..
References
Avanzato, Matson, Seifert, Pryce, Williamson et al., Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer, Cell, doi:10.1016/j.cell.2020.10.049
Baker, Mahmud, Miller, Rajeev, Rasambainarivo et al., Infectious disease in an era of global change, Nat. Rev. Microbiol, doi:10.1038/s41579-021-00639-z
Bartoletti, Azap, Barac, Ben Selma, Ergonul et al., Management of COVID-19 in immunocompromised patients: an European Society of Clinical Microbiology and Infectious Diseases consensus document, Clin. Microbiol. Infect, doi:10.1016/j.cmi.2025.05.032
Beaulieu, Gaymard, Massonnaud, Peiffer-Smadja, Bouscambert-Duchamp et al., Antiviral effect of Evusheld in COVID-19 hospitalized patients infected with pre-Omicron or Omicron variants: a modelling analysis of the randomized DisCoVeRy trial, J. Antimicrob. Chemother, doi:10.1093/jac/dkae301
Bruel, Hadjadj, Maes, Planas, Seve et al., Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies, Nat. Med, doi:10.1038/s41591-022-01792-5
Bruel, Ste ´ Fic, Nguyen, Toniutti, Staropoli et al., Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies, Cell Rep. Med, doi:10.1016/j.xcrm.2022.100850
Bruel, Vrignaud, Porrot, Staropoli, Planas et al., Sotrovimab therapy elicits antiviral activities against Omicron BQ.1.1 and XBB.1.5 in sera of immunocompromised patients, Med, doi:10.1016/j.medj.2023.07.007
Casadevall, Focosi, SARS-CoV-2 variants resistant to monoclonal antibodies in immunocompromised patients constitute a public health concern, J. Clin. Investig, doi:10.1172/jci168603
Cesaro, Ljungman, Mikulska, Hirsch, Navarro et al., Post-pandemic recommendations for the management of COVID-19 in patients with haematological malignancies or undergoing cellular therapy, from the European Conference on Infections in Leukaemia (ECIL-10), Leukemia, doi:10.1038/s41375-025-02649-9
Chen, Kaku, Okumura, Uriu, Zhu et al., Virological characteristics of the SARS-CoV-2 LP.8.1 variant, Lancet Infect. Dis, doi:10.1016/s1473-3099(25)00079-9
Choi, Choudhary, Regan, Sparks, Padera et al., Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host, N. Engl. J. Med, doi:10.1056/nejmc2031364
Corey, Beyrer, Cohen, Michael, Bedford et al., SARS-CoV-2 Variants in Patients with Immunosuppression, N. Engl. J. Med, doi:10.1056/nejmsb2104756
Dufloo, Grzelak, Staropoli, Madec, Tondeur et al., Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies, Cell Rep. Med, doi:10.1016/j.xcrm.2021.100275
Evans, Dube, Lu, Yates, Arnetorp et al., Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study, Lancet Reg. Health. Eur, doi:10.1016/j.lanepe.2023.100747
Focosi, Casadevall, Sipavibart: when a success changes into a failure, Lancet Infect. Dis, doi:10.1016/s1473-3099(24)00812-0
Focosi, Mcconnell, Sullivan, Casadevall, Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment, Drug Resist. Updat, doi:10.1016/j.drup.2023.100991
Fountain-Jones, Vanhaeften, Williamson, Maskell, Chua et al., Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study, Lancet Microbe, doi:10.1016/s2666-5247(23)00393-2
Gottlieb, Vaca, Paredes, Mera, Webb et al., Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients, N. Engl. J. Med, doi:10.1056/nejmoa2116846
Gupta, Gonzalez-Rojas, Juarez, Crespo Casal, Moya et al., Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab, N. Engl. J. Med, doi:10.1056/nejmoa2107934
Gupta, Konnova, Smet, Berkell, Savoldi et al., Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments, J. Clin. Investig, doi:10.1172/jci166032
Hammond, Leister-Tebbe, Gardner, Abreu, Bao et al., Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19, N. Engl. J. Med, doi:10.1056/nejmoa2118542
Kemp, Collier, Datir, Ferreira, Gayed et al., SARS-CoV-2 evolution during treatment of chronic infection, Nature, doi:10.1038/s41586-021-03291-y
Kolmogorov, Billingsley, Mastoras, Meredith, Monlong et al., Scalable Nanopore sequencing of human genomes provides a comprehensive view of haplotype-resolved variation and methylation, Nat. Methods, doi:10.1038/s41592-023-01993-x
Leducq, Zafilaza, Fauchois, Ghidaoui, Sayon et al., Spike Protein Genetic Evolution in Patients at High Risk of Severe Coronavirus Disease 2019 Treated by Monoclonal Antibodies, J. Infect. Dis, doi:10.1093/infdis/jiad523
Leston, Elson, Ordo ´ N ˜ Ez-Mena, Kar, Whitaker et al., Disparities in COVID-19 mortality amongst the immunosuppressed: A systematic review and meta-analysis for enhanced disease surveillance, J. Infect, doi:10.1016/j.jinf.2024.01.009
Li, Choudhary, Regan, Boucau, Nathan et al., SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency, Sci. Transl. Med, doi:10.1126/scitranslmed.adk1599
Mahmoud, Huang, Garimella, Audano, Wan et al., Utility of long-read sequencing for All of Us, Nat. Commun, doi:10.1038/s41467-024-44804-3
Martin-Blondel, Marcelin, Soulie, Kaisaridi, Lusivika-Nzinga et al., Group 1 kit Meso Scale Discovery Custom assay S-PLEX assay #1 Meso Scale Discovery Custom assay S-PLEX assay #2 Meso Scale Discovery Custom assay R-PLEX assay Meso Scale Discovery Custom assay IFNγ ELISPOT kit Medix Biochemica Diaclone 856.051 TaqPath TM COVID-19 RT-PCR assay ThermoFisher Cat# A51738 xGen TM ARTIC nCoV-2019 Amplicon Panel v4.1 Integrated DNA Technologies Cat#10011442 Experimental Models: Cell lines 293T ATCC Cat#CRL-3216 U2OS cells ATCC Cat#HTB-96 Software and Algorithms Harmony High-Content Imaging and Analysis Software PerkinElmer Cat#, J Infection, doi:10.1016/j.jinf.2022.04.010
Martin-Blondel, Marcelin, Soulie, Kaisaridi, Lusivika-Nzinga et al., Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA, J. Infect, doi:10.1016/j.jinf.2022.06.033
Montgomery, Hobbs, Padilla, Arbetter, Templeton et al., Efficacy and safety of intramuscular administration of tixagevimab-cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial, Lancet Respir. Med, doi:10.1016/s2213-2600(22)00180-1
Pantaleo, Correia, Fenwick, Joo, Perez, Antibodies to combat viral infections: development strategies and progress, Nat. Rev. Drug Discov, doi:10.1038/s41573-022-00495-3
Planas, Bruel, Grzelak, Guivel-Benhassine, Staropoli et al., Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies, Nat. Med, doi:10.1038/s41591-021-01318-5
Planas, Saunders, Maes, Guivel-Benhassine, Planchais et al., Considerable escape of SARS-CoV-2 Omicron to antibody neutral-ization, Nature, doi:10.1038/s41586-021-04389-z
Planas, Staropoli, Planchais, Yab, Jeyarajah et al., Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP.3.3 From Approved Monoclonal Antibodies, Pathog. Immun, doi:10.20411/pai.v10i1.752
Planas, Veyer, Baidaliuk, Staropoli, Guivel-Benhassine et al., Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization, Nature, doi:10.1038/s41586-021-03777-9
Schmidt, Narayan, Li, Kaku, Brown et al., Antibody-mediated protection against symptomatic COVID-19 can be achieved at low serum neutralizing titers, Sci. Transl. Med, doi:10.1126/scitranslmed.adg2783
Stadler, Chai, Schlub, Cromer, Khan et al., Determinants of passive antibody efficacy in SARS-CoV-2 infection: a systematic review and meta-analysis, Lancet Microbe, doi:10.1016/s2666-5247(23)00194-5
Weinreich, Sivapalasingam, Norton, Ali, Gao et al., RE-GEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19, N. Engl. J. Med, doi:10.1056/nejmoa2108163
Zheng, Green, Tazare, Curtis, Fisher et al., Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform, BMJ, doi:10.1136/bmj-2022-071932
Zheng, Li, Su, Leung, Lam et al., Symphonizing pileup and full-alignment for deep learning-based longread variant calling, Nat. Comput. Sci, doi:10.1038/s43588-022-00387-x
DOI record:
{
"DOI": "10.1016/j.isci.2025.114226",
"ISSN": [
"2589-0042"
],
"URL": "http://dx.doi.org/10.1016/j.isci.2025.114226",
"alternative-id": [
"S2589004225024873"
],
"article-number": "114226",
"assertion": [
{
"label": "This article is maintained by",
"name": "publisher",
"value": "Elsevier"
},
{
"label": "Article Title",
"name": "articletitle",
"value": "Viral clearance and escape during therapy of COVID-19 outpatients: A prospective cohort study"
},
{
"label": "Journal Title",
"name": "journaltitle",
"value": "iScience"
},
{
"label": "CrossRef DOI link to publisher maintained version",
"name": "articlelink",
"value": "https://doi.org/10.1016/j.isci.2025.114226"
},
{
"label": "Content Type",
"name": "content_type",
"value": "article"
},
{
"label": "Copyright",
"name": "copyright",
"value": "© 2025 The Author(s). Published by Elsevier Inc."
}
],
"author": [
{
"affiliation": [],
"family": "Martin-Blondel",
"given": "Guillaume",
"sequence": "first"
},
{
"affiliation": [],
"family": "Burgat",
"given": "Paul",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leducq",
"given": "Valentin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Porrot",
"given": "Françoise",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cottignies-Calamarte",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Cruz",
"given": "Alejandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dorival",
"given": "Céline",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romieu-Mourez",
"given": "Raphaelle",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chaubet",
"given": "Camille",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cazaentre",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boumaza",
"given": "Xavier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Richier",
"given": "Quentin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gaborit",
"given": "Benjamin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Coustilleres",
"given": "Francois",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Dubée",
"given": "Vincent",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ader",
"given": "Florence",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yordanov",
"given": "Youri",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Schwartz",
"given": "Olivier",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marcelin",
"given": "Anne-Geneviève",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lusivika-Nzinga",
"given": "Clovis",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Carrat",
"given": "Fabrice",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Soulié",
"given": "Cathia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liblau",
"given": "Roland",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bruel",
"given": "Timothée",
"sequence": "additional"
}
],
"container-title": "iScience",
"container-title-short": "iScience",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"cell.com",
"elsevier.com",
"sciencedirect.com"
]
},
"created": {
"date-parts": [
[
2025,
11,
26
]
],
"date-time": "2025-11-26T16:50:16Z",
"timestamp": 1764175816000
},
"deposited": {
"date-parts": [
[
2026,
1,
20
]
],
"date-time": "2026-01-20T18:29:13Z",
"timestamp": 1768933753000
},
"funder": [
{
"DOI": "10.13039/501100014630",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100014630",
"id-type": "DOI"
}
],
"name": "Ministère des Solidarités et de la Santé"
},
{
"DOI": "10.13039/501100011045",
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100011045",
"id-type": "DOI"
}
],
"name": "Ministère de l'Enseignement supérieur, de la Recherche et de l'Innovation"
},
{
"DOI": "10.13039/501100001665",
"award": [
"ANR-23-CE15-0039-01"
],
"award-info": [
{
"award-number": [
"ANR-23-CE15-0039-01"
]
}
],
"doi-asserted-by": "publisher",
"id": [
{
"asserted-by": "publisher",
"id": "10.13039/501100001665",
"id-type": "DOI"
}
],
"name": "French National Research Agency"
}
],
"indexed": {
"date-parts": [
[
2026,
1,
21
]
],
"date-time": "2026-01-21T07:21:01Z",
"timestamp": 1768980061393,
"version": "3.49.0"
},
"is-referenced-by-count": 0,
"issue": "12",
"issued": {
"date-parts": [
[
2025,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-print": {
"date-parts": [
[
2025,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://www.elsevier.com/tdm/userlicense/1.0/",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
12,
1
]
],
"date-time": "2025-12-01T00:00:00Z",
"timestamp": 1764547200000
}
},
{
"URL": "https://www.elsevier.com/legal/tdmrep-license",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
12,
1
]
],
"date-time": "2025-12-01T00:00:00Z",
"timestamp": 1764547200000
}
},
{
"URL": "http://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2025,
11,
21
]
],
"date-time": "2025-11-21T00:00:00Z",
"timestamp": 1763683200000
}
}
],
"link": [
{
"URL": "https://api.elsevier.com/content/article/PII:S2589004225024873?httpAccept=text/xml",
"content-type": "text/xml",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://api.elsevier.com/content/article/PII:S2589004225024873?httpAccept=text/plain",
"content-type": "text/plain",
"content-version": "vor",
"intended-application": "text-mining"
}
],
"member": "78",
"original-title": [],
"page": "114226",
"prefix": "10.1016",
"published": {
"date-parts": [
[
2025,
12
]
]
},
"published-print": {
"date-parts": [
[
2025,
12
]
]
},
"publisher": "Elsevier BV",
"reference": [
{
"article-title": "Impact of COVID-19 on immunocompromised populations during the Omicron era: insights from the observational population-based INFORM study",
"author": "Evans",
"journal-title": "Lancet Reg. Health. Eur.",
"key": "10.1016/j.isci.2025.114226_bib1",
"volume": "35",
"year": "2023"
},
{
"DOI": "10.1016/j.jinf.2024.01.009",
"article-title": "Disparities in COVID-19 mortality amongst the immunosuppressed: A systematic review and meta-analysis for enhanced disease surveillance",
"author": "Leston",
"doi-asserted-by": "crossref",
"journal-title": "J. Infect.",
"key": "10.1016/j.isci.2025.114226_bib2",
"volume": "88",
"year": "2024"
},
{
"DOI": "10.1126/scitranslmed.adk1599",
"article-title": "SARS-CoV-2 viral clearance and evolution varies by type and severity of immunodeficiency",
"author": "Li",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Transl. Med.",
"key": "10.1016/j.isci.2025.114226_bib3",
"volume": "16",
"year": "2024"
},
{
"DOI": "10.1016/j.cell.2020.10.049",
"article-title": "Case Study: Prolonged Infectious SARS-CoV-2 Shedding from an Asymptomatic Immunocompromised Individual with Cancer",
"author": "Avanzato",
"doi-asserted-by": "crossref",
"first-page": "1901",
"journal-title": "Cell",
"key": "10.1016/j.isci.2025.114226_bib4",
"volume": "183",
"year": "2020"
},
{
"DOI": "10.1056/NEJMc2031364",
"article-title": "Persistence and Evolution of SARS-CoV-2 in an Immunocompromised Host",
"author": "Choi",
"doi-asserted-by": "crossref",
"first-page": "2291",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2025.114226_bib5",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1038/s41586-021-03291-y",
"article-title": "SARS-CoV-2 evolution during treatment of chronic infection",
"author": "Kemp",
"doi-asserted-by": "crossref",
"first-page": "277",
"journal-title": "Nature",
"key": "10.1016/j.isci.2025.114226_bib6",
"volume": "592",
"year": "2021"
},
{
"DOI": "10.1056/NEJMsb2104756",
"article-title": "SARS-CoV-2 Variants in Patients with Immunosuppression",
"author": "Corey",
"doi-asserted-by": "crossref",
"first-page": "562",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2025.114226_bib7",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S2666-5247(23)00194-5",
"article-title": "Determinants of passive antibody efficacy in SARS-CoV-2 infection: a systematic review and meta-analysis",
"author": "Stadler",
"doi-asserted-by": "crossref",
"first-page": "e883",
"journal-title": "Lancet Microbe",
"key": "10.1016/j.isci.2025.114226_bib8",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1093/jac/dkae301",
"article-title": "Antiviral effect of Evusheld in COVID-19 hospitalized patients infected with pre-Omicron or Omicron variants: a modelling analysis of the randomized DisCoVeRy trial",
"author": "Beaulieu",
"doi-asserted-by": "crossref",
"first-page": "2887",
"journal-title": "J. Antimicrob. Chemother.",
"key": "10.1016/j.isci.2025.114226_bib9",
"volume": "79",
"year": "2024"
},
{
"DOI": "10.1136/bmj-2022-071932",
"article-title": "Comparative effectiveness of sotrovimab and molnupiravir for prevention of severe covid-19 outcomes in patients in the community: observational cohort study with the OpenSAFELY platform",
"author": "Zheng",
"doi-asserted-by": "crossref",
"journal-title": "BMJ",
"key": "10.1016/j.isci.2025.114226_bib10",
"volume": "379",
"year": "2022"
},
{
"DOI": "10.1172/JCI168603",
"article-title": "SARS-CoV-2 variants resistant to monoclonal antibodies in immunocompromised patients constitute a public health concern",
"author": "Casadevall",
"doi-asserted-by": "crossref",
"journal-title": "J. Clin. Investig.",
"key": "10.1016/j.isci.2025.114226_bib11",
"volume": "133",
"year": "2023"
},
{
"DOI": "10.1016/j.drup.2023.100991",
"article-title": "Analysis of SARS-CoV-2 mutations associated with resistance to therapeutic monoclonal antibodies that emerge after treatment",
"author": "Focosi",
"doi-asserted-by": "crossref",
"journal-title": "Drug Resist. Updat.",
"key": "10.1016/j.isci.2025.114226_bib12",
"volume": "71",
"year": "2023"
},
{
"DOI": "10.1093/infdis/jiad523",
"article-title": "Spike Protein Genetic Evolution in Patients at High Risk of Severe Coronavirus Disease 2019 Treated by Monoclonal Antibodies",
"author": "Leducq",
"doi-asserted-by": "crossref",
"first-page": "1341",
"journal-title": "J. Infect. Dis.",
"key": "10.1016/j.isci.2025.114226_bib13",
"volume": "229",
"year": "2024"
},
{
"DOI": "10.1038/s41579-021-00639-z",
"article-title": "Infectious disease in an era of global change",
"author": "Baker",
"doi-asserted-by": "crossref",
"first-page": "193",
"journal-title": "Nat. Rev. Microbiol.",
"key": "10.1016/j.isci.2025.114226_bib14",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.1038/s41573-022-00495-3",
"article-title": "Antibodies to combat viral infections: development strategies and progress",
"author": "Pantaleo",
"doi-asserted-by": "crossref",
"first-page": "676",
"journal-title": "Nat. Rev. Drug Discov.",
"key": "10.1016/j.isci.2025.114226_bib15",
"volume": "21",
"year": "2022"
},
{
"DOI": "10.1172/JCI166032",
"article-title": "Host immunological responses facilitate development of SARS-CoV-2 mutations in patients receiving monoclonal antibody treatments",
"author": "Gupta",
"doi-asserted-by": "crossref",
"journal-title": "J. Clin. Investig.",
"key": "10.1016/j.isci.2025.114226_bib16",
"volume": "133",
"year": "2023"
},
{
"DOI": "10.1016/j.medj.2023.07.007",
"article-title": "Sotrovimab therapy elicits antiviral activities against Omicron BQ.1.1 and XBB.1.5 in sera of immunocompromised patients",
"author": "Bruel",
"doi-asserted-by": "crossref",
"first-page": "664",
"journal-title": "Med",
"key": "10.1016/j.isci.2025.114226_bib17",
"volume": "4",
"year": "2023"
},
{
"DOI": "10.1126/scitranslmed.adg2783",
"article-title": "Antibody-mediated protection against symptomatic COVID-19 can be achieved at low serum neutralizing titers",
"author": "Schmidt",
"doi-asserted-by": "crossref",
"journal-title": "Sci. Transl. Med.",
"key": "10.1016/j.isci.2025.114226_bib18",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.1038/s41375-025-02649-9",
"article-title": "Post-pandemic recommendations for the management of COVID-19 in patients with haematological malignancies or undergoing cellular therapy, from the European Conference on Infections in Leukaemia (ECIL-10)",
"author": "Cesaro",
"doi-asserted-by": "crossref",
"first-page": "2061",
"journal-title": "Leukemia",
"key": "10.1016/j.isci.2025.114226_bib19",
"volume": "39",
"year": "2025"
},
{
"DOI": "10.1016/j.cmi.2025.05.032",
"article-title": "Management of COVID-19 in immunocompromised patients: an European Society of Clinical Microbiology and Infectious Diseases consensus document",
"author": "Bartoletti",
"doi-asserted-by": "crossref",
"first-page": "1655",
"journal-title": "Clin. Microbiol. Infect.",
"key": "10.1016/j.isci.2025.114226_bib20",
"volume": "31",
"year": "2025"
},
{
"DOI": "10.1016/S2666-5247(23)00393-2",
"article-title": "Effect of molnupiravir on SARS-CoV-2 evolution in immunocompromised patients: a retrospective observational study",
"author": "Fountain-Jones",
"doi-asserted-by": "crossref",
"first-page": "e452",
"journal-title": "Lancet Microbe",
"key": "10.1016/j.isci.2025.114226_bib21",
"volume": "5",
"year": "2024"
},
{
"article-title": "Escape of SARS-CoV-2 Variants KP.1.1, LB.1, and KP.3.3 From Approved Monoclonal Antibodies. Pathog",
"author": "Planas",
"first-page": "1",
"journal-title": "Immun",
"key": "10.1016/j.isci.2025.114226_bib22",
"volume": "10",
"year": "2024"
},
{
"DOI": "10.1016/S1473-3099(25)00079-9",
"article-title": "Virological characteristics of the SARS-CoV-2 LP.8.1 variant",
"author": "Chen",
"doi-asserted-by": "crossref",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.isci.2025.114226_bib23",
"volume": "25",
"year": "2025"
},
{
"DOI": "10.1016/S1473-3099(24)00812-0",
"article-title": "Sipavibart: when a success changes into a failure",
"author": "Focosi",
"doi-asserted-by": "crossref",
"first-page": "713",
"journal-title": "Lancet Infect. Dis.",
"key": "10.1016/j.isci.2025.114226_bib24",
"volume": "25",
"year": "2025"
},
{
"DOI": "10.1016/j.jinf.2022.06.033",
"article-title": "Sotrovimab to prevent severe COVID-19 in high-risk patients infected with Omicron BA.2",
"author": "Martin-Blondel",
"doi-asserted-by": "crossref",
"first-page": "e104",
"journal-title": "J. Infect.",
"key": "10.1016/j.isci.2025.114226_bib25",
"volume": "85",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2108163",
"article-title": "REGEN-COV Antibody Combination and Outcomes in Outpatients with Covid-19",
"author": "Weinreich",
"doi-asserted-by": "crossref",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2025.114226_bib26",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2107934",
"article-title": "Early Treatment for Covid-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab",
"author": "Gupta",
"doi-asserted-by": "crossref",
"first-page": "1941",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2025.114226_bib27",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1016/S2213-2600(22)00180-1",
"article-title": "Efficacy and safety of intramuscular administration of tixagevimab–cilgavimab for early outpatient treatment of COVID-19 (TACKLE): a phase 3, randomised, double-blind, placebo-controlled trial",
"author": "Montgomery",
"doi-asserted-by": "crossref",
"first-page": "985",
"journal-title": "Lancet Respir. Med.",
"key": "10.1016/j.isci.2025.114226_bib28",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with Covid-19",
"author": "Hammond",
"doi-asserted-by": "crossref",
"first-page": "1397",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2025.114226_bib29",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2116846",
"article-title": "Early Remdesivir to Prevent Progression to Severe Covid-19 in Outpatients",
"author": "Gottlieb",
"doi-asserted-by": "crossref",
"first-page": "305",
"journal-title": "N. Engl. J. Med.",
"key": "10.1016/j.isci.2025.114226_bib30",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1038/s41467-024-44804-3",
"article-title": "Utility of long-read sequencing for All of Us",
"author": "Mahmoud",
"doi-asserted-by": "crossref",
"first-page": "837",
"journal-title": "Nat. Commun.",
"key": "10.1016/j.isci.2025.114226_bib31",
"volume": "15",
"year": "2024"
},
{
"DOI": "10.1038/s41592-023-01993-x",
"article-title": "Scalable Nanopore sequencing of human genomes provides a comprehensive view of haplotype-resolved variation and methylation",
"author": "Kolmogorov",
"doi-asserted-by": "crossref",
"first-page": "1483",
"journal-title": "Nat. Methods",
"key": "10.1016/j.isci.2025.114226_bib32",
"volume": "20",
"year": "2023"
},
{
"DOI": "10.1038/s43588-022-00387-x",
"article-title": "Symphonizing pileup and full-alignment for deep learning-based long-read variant calling",
"author": "Zheng",
"doi-asserted-by": "crossref",
"first-page": "797",
"journal-title": "Nat. Comput. Sci.",
"key": "10.1016/j.isci.2025.114226_bib33",
"volume": "2",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-04389-z",
"article-title": "Considerable escape of SARS-CoV-2 Omicron to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "671",
"journal-title": "Nature",
"key": "10.1016/j.isci.2025.114226_bib34",
"volume": "602",
"year": "2022"
},
{
"DOI": "10.1038/s41586-021-03777-9",
"article-title": "Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "276",
"journal-title": "Nature",
"key": "10.1016/j.isci.2025.114226_bib35",
"volume": "596",
"year": "2021"
},
{
"DOI": "10.1038/s41591-022-01792-5",
"article-title": "Serum neutralization of SARS-CoV-2 Omicron sublineages BA.1 and BA.2 in patients receiving monoclonal antibodies",
"author": "Bruel",
"doi-asserted-by": "crossref",
"first-page": "1297",
"journal-title": "Nat. Med.",
"key": "10.1016/j.isci.2025.114226_bib36",
"volume": "28",
"year": "2022"
},
{
"article-title": "Longitudinal analysis of serum neutralization of SARS-CoV-2 Omicron BA.2, BA.4, and BA.5 in patients receiving monoclonal antibodies",
"author": "Bruel",
"journal-title": "Cell Rep. Med.",
"key": "10.1016/j.isci.2025.114226_bib37",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1038/s41591-021-01318-5",
"article-title": "Sensitivity of infectious SARS-CoV-2 B.1.1.7 and B.1.351 variants to neutralizing antibodies",
"author": "Planas",
"doi-asserted-by": "crossref",
"first-page": "917",
"journal-title": "Nat. Med.",
"key": "10.1016/j.isci.2025.114226_bib38",
"volume": "27",
"year": "2021"
},
{
"article-title": "Asymptomatic and symptomatic SARS-CoV-2 infections elicit polyfunctional antibodies",
"author": "Dufloo",
"journal-title": "Cell Rep. Med.",
"key": "10.1016/j.isci.2025.114226_bib39",
"volume": "2",
"year": "2021"
},
{
"DOI": "10.1016/j.jinf.2022.04.010",
"article-title": "Outcome of very high-risk patients treated by Sotrovimab for mild-to-moderate COVID-19 Omicron, a prospective cohort study (the ANRS 0003S COCOPREV study)",
"author": "Martin-Blondel",
"doi-asserted-by": "crossref",
"first-page": "e101",
"journal-title": "J Infection",
"key": "10.1016/j.isci.2025.114226_bib40",
"volume": "84",
"year": "2022"
}
],
"reference-count": 40,
"references-count": 40,
"relation": {},
"resource": {
"primary": {
"URL": "https://linkinghub.elsevier.com/retrieve/pii/S2589004225024873"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Viral clearance and escape during therapy of COVID-19 outpatients: A prospective cohort study",
"type": "journal-article",
"update-policy": "https://doi.org/10.1016/elsevier_cm_policy",
"volume": "28"
}
