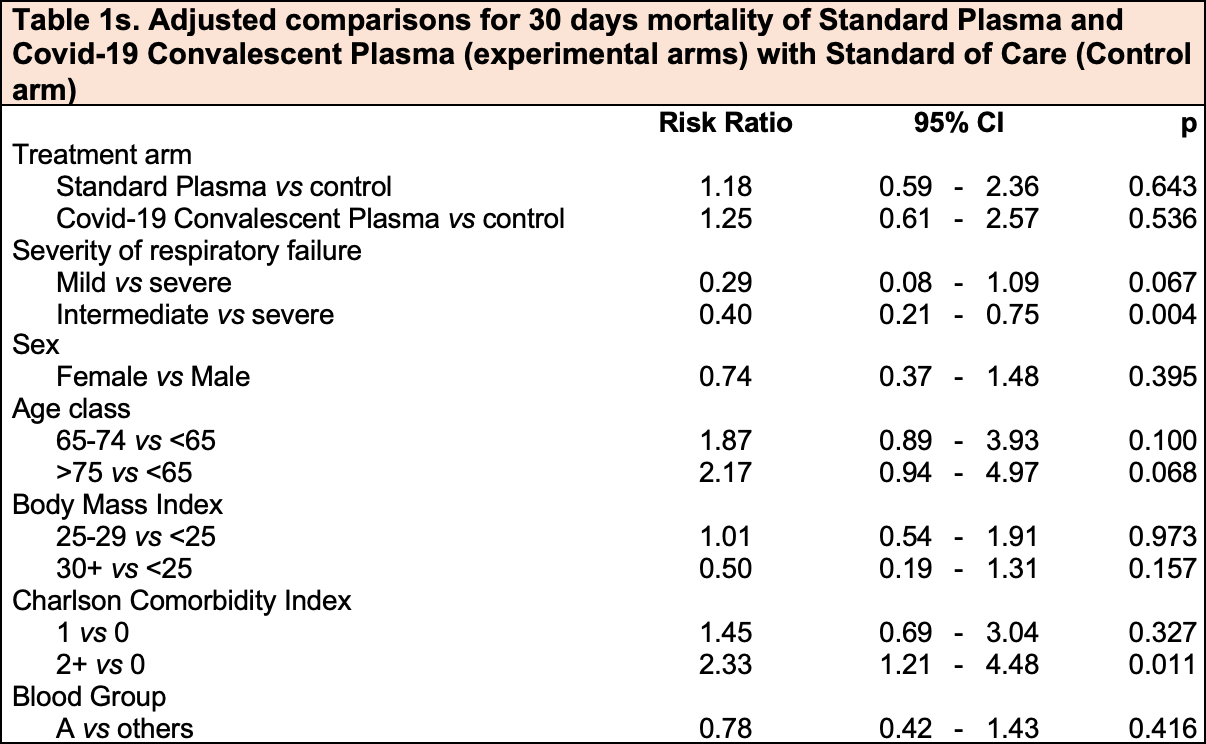
Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: short and long-term effects. A three-arm randomized controlled clinical trial
et al., BMC Infectious Diseases, doi:10.1186/s12879-022-07716-5, PLACO COVID, NCT04428021, Nov 2022
RCT 180 hospitalized COVID-19 patients with respiratory impairment in Italy showing no significant improvement in mortality or mechanical ventilation with either standard plasma or COVID-19 convalescent plasma compared to standard of care.
|
risk of death, 25.0% higher, RR 1.25, p = 0.54, treatment 14 of 60 (23.3%), control 12 of 60 (20.0%), adjusted per study, day 30.
|
|
risk of death/intubation, 10.0% higher, RR 1.10, p = 0.76, treatment 17 of 59 (28.8%), control 14 of 56 (25.0%), day 30.
|
|
time to viral-, 6.4% higher, relative time 1.06, p = 0.76, treatment 60, control 60, inverted to make RR<1 favor treatment.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Manzini et al., 22 Nov 2022, Double Blind Randomized Controlled Trial, Italy, peer-reviewed, median age 66.6, 54 authors, study period June 2020 - August 2021, trial NCT04428021 (history) (PLACO COVID).
Contact: pmanzini@cittadellasalute.to.it (corresponding author).
Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: short and long-term effects. A three-arm randomized controlled clinical trial
BMC Infectious Diseases, doi:10.1186/s12879-022-07716-5
Background: The efficacy of early treatment with convalescent plasma in patients with COVID-19 is debated. Nothing is known about the potential effect of other plasma components other than anti-SARS-CoV-2 antibodies. Methods: To determine whether convalescent or standard plasma would improve outcomes for adults in early phase of Covid19 respiratory impairment we designed this randomized, three-arms, clinical trial (PLACO COVID) blinded on interventional arms that was conducted from June 2020 to August 2021. It was a multicentric trial at 19 Italian hospitals. We enrolled 180 hospitalized adult patients with COVID-19 pneumonia within 5 days from the onset of respiratory distress. Patients were randomly assigned in a 1:1:1 ratio to standard of care (n = 60) or standard of care + three units of standard plasma (n = 60) or standard of care + three units of high-titre convalescent plasma (n = 60) administered on days 1, 3, 5 after randomization. Primary outcome was 30-days mortality. Secondary outcomes were: incidence of mechanical ventilation or death at day 30, 6-month mortality, proportion of days with mechanical ventilation on total length of hospital stay, IgG anti-SARS-CoV-2 seroconversion, viral clearance from plasma and respiratory
Abbreviations
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12879-022-07716-5.
Additional file 1. Supplementary appendix.
Author contributions PMM is the Chief Investigator; she conceived the study, led the proposal and protocol development and wrote the manuscript. GCi, FGDR, CG, SDA, FS, ACas, ML, GCa, OG, AMB, AT, LB, LS, Cav, MPri, FE, contributed to study design and to development of the proposal. GCi was the lead trial methodologist. FS, ACas were trial metodologists and they analyzed results. FD, LL, designed logistic organization for CCP preparation and distribution. TF, HH, CP, MP, PO, ITS, RG, RF, PB, AN, LMa, FPM, SR, AB, AR, MMi, GD, FCas, AV, RR, AP, DO, RA, BLS, IG, ACi, RLG, ADR, LC, TB, OC, KG, FN, MT, PO, GBa, LMaz, VB, MPr, LAL, MGC, MGia, LR, DDM, SMa, MMo, GSe, PSp, GGia organized donors' selection and recruitment and plasma and CCP collection in their hospitals. TF, HH, CP, GD, GGu, CF, DMLV, FPol, SLe, ChL, DRo, SZ, MeMa, IA, SG, RBa, VN, CA, MCP, ST, MCa, GSt, VlG, organized patients' data collection for their centers. BM, GSc, CP were responsible for coordinating Hospital organization for the trial. RC, VG, FD, LL, FP, MA, CC, MGM, organized and performed all donors and patients serological and RT-PCR tests. GC, GL, CSc, FF, CSo, MR, CG, organized plasma and CCP fractionation, treatment and stockage. GR, TDA, CC, SN, VaG, LP, FPio, GGiu, organized CCP and plasma blinding..
References
Abolghasemi, Eshghi, Cheraghali, Fooladi, Moghaddam et al., Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study, Transfus Apher Sci
Agarwal, Mukherjee, Kumar, Chatterjee, Bhatnagar et al., Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial), BMJ
Alqahtani, Abdulrahman, Almadani, Alali, Zamrooni et al., Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease, Infect Dis
Arrigo, Hospital, Saints Anthony and Biagio and
Avendaño-Solà, Ramos-Martínez, Muñez-Rubio, Ruiz-Antorán, De Molina et al., Convalescent plasma for COVID-19: a multicenter, randomized clinical trial, Infect Dis
Axfors, Janiaud, Schmitt, Van't Hooft, Smith et al., Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials, BMC Infect Dis
Bajpai, Kumar, Maheshwari, Chhabra, Gupta, Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial, Infect Dis
Balcells, Rojas, Corre, Martínez-Valdebenito, Ceballos et al., Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial, PLoS Med
Bégin, Callum, Jamula, Cook, Heddle et al., Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial, Nat Med, doi:10.1038/s41591-021-01488-2
Chen, Lan, Yuan, Deng, Li et al., Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity, Emerg Microbes Infect
Chen, Zhao, Qu, Chen, Xiong et al., Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019, Clin Infect Dis
Cheng, Wong, Soo, Wong, Lee et al., Use of convalescent plasma therapy in SARS patients in Hong Kong, Eur J Clin Microbiol Infect Dis
Diseases, Croce, Cuneo, District, Cuneo, 27 Infectious Diseases Unit
Duan, Liu, Li, Zhang, Yu et al., Effectiveness of convalescent plasma therapy in severe COVID-19 patients, Proc Natl Acad Sci
Eberhardt, Meyer-Schwickerath, Heger, Knops, Lehmann et al., RNAemia corresponds to disease severity and antibody response in hospitalized COVID-19 patients, Viruses
Gharbharan, Jordans, Geurtsvankessel, Hollander, Karim et al., Convalescent Plasma for COVID-19. A randomized clinical trial, Infect Dis
Hagman, Hedenstierna, Gille-Johnson, Hammas, Grabbe et al., Severe acute respiratory syndrome coronavirus 2 RNA in serum as predictor of severe outcome in coronavirus disease 2019: a retrospective cohort study, Clin Infect Dis
Hegerova, Gooley, Sweerus, Maree, Bailey et al., Use of convalescent plasma in hospitalized patients with COVID-19: case series, Blood
Hogan, Stevens, Sahoo, Huang, Garamani et al., High frequency of SARS-CoV-2 RNAemia and association with severe disease, Clin Infect Dis
Hung, To, Lee, Lee, Yan, Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection, Clin Infect Dis
Joyner, Bruno, Klassen, Kunze, Johnson et al., Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients, Mayo Clin Proc
Joyner, Carter, Senefeld, Klassen, Mills et al., Convalescent plasma antibody levels and the risk of death from COVID-19, N Engl J Med
Joyner, Senefeld, Klassen, Mills, Johnson et al., Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience, Infect Dis
Ko, Seok, Cho, Ha, Baek et al., Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience, Antivir Ther
Korley, Durkalski-Mauldin, Yeatts, Schulman, Davenport et al., Early convalescent plasma for high-risk outpatients with COVID-19, N Engl J Med
Li, Zhang, Hu, Tong, Zheng et al., Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial, JAMA
Libster, Marc, Wappner, Coviello, Bianchi et al., Early high-titer plasma therapy to prevent severe COVID-19 in older adults, N Engl J Med
Liu, Lin, Baine, Wajnberg, Gumprecht et al., Convalescent plasma treatment of severe COVID-19: a matched control study, Infect Dis
Maiztegui, Fernandez, De Damilano, Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome, Lancet Lond Engl
Medicine, Edoardo, Hospital, Internal and Emergency Medicine
Medicine, Hospital, Immunohematology and Transfusion Medicine
Medicine, Hospital, Ovada, 87 Internal medicine
Menichetti, Popoli, Puopolo, Alegiani, Tiseo et al., Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial, JAMA Netw Open
O'donnell, Grinsztejn, Cummings, Justman, Lamb et al., A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19, Infect Dis
Odutayo, Gryaznov, Copsey, Monk, Speich et al., Design, analysis and reporting of multi-arm trials and strategies to address multiple testing, Int J Epidemiol
Pathak, Convalescent plasma is ineffective for covid-19, BMJ
Perotti, Baldanti, Bruno, Fante, Seminari et al., Mortality reduction in 46 severe COVID-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter interventional trial, Infect Dis
Ray, Paul, Bandopadhyay, 'rozario, Sarif et al., Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial, Infect Dis
Salazar, Christensen, Graviss, Nguyen, Castillo et al., Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality, Am J Pathol
Service, Hospital, Verduno, Italy. 92 Medical Emergency Division
Shen, Wang, Zhao, Yang, Li et al., Treatment of 5 critically ill patients with covid-19 with convalescent plasma, JAMA
Simonovich, Pratx, Scibona, Beruto, Vallone et al., A randomized trial of convalescent plasma in COVID-19 severe pneumonia, N Engl J Med
Unit, Anthony, Arrigo, Hospital, Internal Medicine, Saints Anthony and Biagio and Cesare Arrigo Alessandria National Hospital
Vco, None
Zhou, Zhong, Guan, Treatment with convalescent plasma for influenza A (H5N1) infection, N Engl J Med
DOI record:
{
"DOI": "10.1186/s12879-022-07716-5",
"ISSN": [
"1471-2334"
],
"URL": "http://dx.doi.org/10.1186/s12879-022-07716-5",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>The efficacy of early treatment with convalescent plasma in patients with COVID-19 is debated. Nothing is known about the potential effect of other plasma components other than anti-SARS-CoV-2 antibodies.\n</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>To determine whether convalescent or standard plasma would improve outcomes for adults in early phase of Covid19 respiratory impairment we designed this randomized, three-arms, clinical trial (PLACO COVID) blinded on interventional arms that was conducted from June 2020 to August 2021. It was a multicentric trial at 19 Italian hospitals. We enrolled 180 hospitalized adult patients with COVID-19 pneumonia within 5 days from the onset of respiratory distress. Patients were randomly assigned in a 1:1:1 ratio to standard of care (n = 60) or standard of care + three units of standard plasma (n = 60) or standard of care + three units of high-titre convalescent plasma (n = 60) administered on days 1, 3, 5 after randomization. Primary outcome was 30-days mortality. Secondary outcomes were: incidence of mechanical ventilation or death at day 30, 6-month mortality, proportion of days with mechanical ventilation on total length of hospital stay, IgG anti-SARS-CoV-2 seroconversion, viral clearance from plasma and respiratory tract samples, and variations in Sequential Organ Failure Assessment score. The trial was analysed according to the intention-to-treat principle.\n</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>180 patients (133/180 [73.9%] males, mean age 66.6 years [IQR 57–73]) were enrolled a median of 8 days from onset of symptoms. At enrollment, 88.9% of patients showed moderate/severe respiratory failure. 30-days mortality was 20% in Control arm, 23% in Convalescent (risk ratio [RR] 1.13; 95% confidence interval [CI], 0.61–2.13, P = 0.694) and 25% in Standard plasma (RR 1.23; 95%CI, 0.63–2.37, P = 0.544). Time to viral clearance from respiratory tract was 21 days for Convalescent, 28 for Standard plasma and 23 in Control arm but differences were not statistically significant. No differences for other secondary endpoints were seen in the three arms. Serious adverse events were reported in 1.7%, 3.3% and 5% of patients in Control, Standard and Convalescent plasma arms respectively.\n</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Neither high-titer Convalescent nor Standard plasma improve outcomes of COVID-19 patients with acute respiratory failure.</jats:p>\n <jats:p><jats:italic>Trial Registration</jats:italic> Clinicaltrials.gov Identifier: NCT04428021. First posted: 11/06/2020</jats:p>\n </jats:sec>",
"alternative-id": [
"7716"
],
"article-number": "879",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "21 February 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "30 August 2022"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "22 November 2022"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "The trial protocol was approved by the Ethical Committee of the coordinating center: Comitato Etico Interaziendale A.O.U. Città della Salute e della Scienza di Torino—A.O. Ordine Mauriziano di Torino—A.S.L. Città di Torino and then by all ethical Committee at each participating center and was conducted following the principles stated in the declaration of Helsinki and the Good Clinical Practice Guidelines. A written informed consent was obtained from all subjects (donors and patients) and/or their legal guardian(s) before enrollment. The informed consent is available in the Study Protocol available in the online version—see Supplementary Information)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare that they have no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Manzini",
"given": "Paola Maria",
"sequence": "first"
},
{
"affiliation": [],
"family": "Ciccone",
"given": "Giovannino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Rosa",
"given": "Francesco Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cavallo",
"given": "Rossana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghisetti",
"given": "Valeria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "D’Antico",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Galassi",
"given": "Claudia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Saccona",
"given": "Fabio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castiglione",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Birocco",
"given": "Nadia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Francisci",
"given": "Tiziana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hu",
"given": "Huijing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pecoraro",
"given": "Clara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Danielle",
"given": "Franca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Labanca",
"given": "Luciana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bordiga",
"given": "Anna Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lorenzi",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camisasca",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giachino",
"given": "Osvaldo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pagliarino",
"given": "Mauro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ottone",
"given": "Piero",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scuvera",
"given": "Ilvana Tiziana Donatella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guaschino",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Freilone",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Berti",
"given": "Pierluigi",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pittaluga",
"given": "Fabrizia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avolio",
"given": "Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Costa",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Raso",
"given": "Samuele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nucci",
"given": "Aurora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milan",
"given": "Massimo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baffa",
"given": "Alessandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russo",
"given": "Alessandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tornello",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Maddalena",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Delios",
"given": "Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marletto",
"given": "Fabio Paolo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Micheli",
"given": "Anna Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mattei",
"given": "Alessio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Baldassano",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Canta",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Russo",
"given": "Maria Luisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bergamo",
"given": "Daniele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vitale",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liccardi",
"given": "Marco Maria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chinaglia",
"given": "Alessandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calcagno",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Converso",
"given": "Marcella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aldieri",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Libanore",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Blangetti",
"given": "Ilaria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Benedetti",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mitola",
"given": "Barbara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scozzari",
"given": "Gitana",
"sequence": "additional"
},
{
"affiliation": [],
"name": "the PLACO COVID Study Group",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castagno",
"given": "Franco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Valfrè",
"given": "Adriano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Rizzioli",
"given": "Gabriella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "D’Amato",
"given": "Teresa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Crocillà",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Naselli",
"given": "Silvana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Granero",
"given": "Valentino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cornagliotto",
"given": "Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lucania",
"given": "Graziella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scaglia",
"given": "Cristiana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ferro",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Solimine",
"given": "Carmela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ricotti",
"given": "Monica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gilestro",
"given": "Cristina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roncato",
"given": "Remigio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Palladino",
"given": "Angela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ongaro",
"given": "Daniela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Poggio",
"given": "Giulia Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chiappero",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pinna",
"given": "Simone Mornese",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scabini",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vischia",
"given": "Federico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gregoretti",
"given": "Maria Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lupia",
"given": "Enrico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Brazzi",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albera",
"given": "Carlo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scaglione",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gallo",
"given": "Valter",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Norbiato",
"given": "Claudio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Albiani",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sini",
"given": "Bruno Lucio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fassiola",
"given": "Andrea",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Locatelli",
"given": "Alessandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Di Perri",
"given": "Giovanni",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Navarra",
"given": "Mauro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gardini",
"given": "Isabella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ciardiello",
"given": "Aurora",
"sequence": "additional"
},
{
"affiliation": [],
"family": "La Grotta",
"given": "Rita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Rosa",
"given": "Anna",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pasquino",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Fiore",
"given": "Gilberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Franza",
"given": "Orietta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Artoni",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meinardi",
"given": "Stefano",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Calosso",
"given": "Liliana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Molino",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Veglio",
"given": "Maria Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Beltramo",
"given": "Tiziana",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Camerini",
"given": "Odetta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giancaspero",
"given": "Karol",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Napoli",
"given": "Franca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perboni",
"given": "Alberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Messa",
"given": "Emanuela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buffolo",
"given": "Fabrizio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pagnozzi",
"given": "Fiammetta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Bertone",
"given": "Stefania",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lutri",
"given": "Lorenzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gravante",
"given": "Umberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sacchetti",
"given": "Petros",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pavan",
"given": "Alessandra",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Castenetto",
"given": "Enzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Novelli",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tucciarone",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ocello",
"given": "Patrizia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Guido",
"given": "Giulia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Frascaroli",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vivenza",
"given": "Daniela Maria Luisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Patti",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lorenzelli",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balduzzi",
"given": "Guido",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ratti",
"given": "Deborah",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Mazzucco",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balbo",
"given": "Valeria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pollis",
"given": "Francesca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Leoncino",
"given": "Sabrina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lupo",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Romano",
"given": "Daniele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ziccardi",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Marmifero",
"given": "Melania",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chichino",
"given": "Guido",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Salio",
"given": "Mario",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aiosa",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Boverio",
"given": "Riccardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avonto",
"given": "Ilaria",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghiotto",
"given": "Sara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Balbo",
"given": "Riccardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Nico",
"given": "Vincenza",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aguzzi",
"given": "Chiara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pellegrino",
"given": "Maria Chiara",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prucca",
"given": "Maristella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Longa",
"given": "Lucia Assunta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Perotti",
"given": "Laura",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Piovano",
"given": "Federica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ambrogio",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Formica",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Monge",
"given": "Elisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Arena",
"given": "Flavia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Barzaghi",
"given": "Nicoletta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tavera",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Canepari",
"given": "Mariaelisa",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Strani",
"given": "Guido",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pomero",
"given": "Fulvio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cianci",
"given": "Maria Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gianarda",
"given": "Mariella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ruscitto",
"given": "Leonardo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "De Martino",
"given": "Daniel",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Macchi",
"given": "Sandro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montagnana",
"given": "Michele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grandinetti",
"given": "Vladimiro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Magnani",
"given": "Silvia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Radin",
"given": "Elisabetta",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pellu",
"given": "Valentina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Meucci",
"given": "Monica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Noè",
"given": "Erika",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Torti",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Montagnani",
"given": "Luca",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Doveri",
"given": "Giulio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Giustetto",
"given": "Gabriella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Avdis",
"given": "Costantino",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prina",
"given": "Marco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Eliantonio",
"given": "Franco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lemut",
"given": "Francesco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Semino",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Spidalieri",
"given": "Palmina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vallino",
"given": "Domenico",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Prota",
"given": "Roberto",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Buono",
"given": "Gabriella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Segala",
"given": "Vincenzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Milia",
"given": "Maria Grazia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aprà",
"given": "Franco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Livigni",
"given": "Sergio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Manno",
"given": "Emilpaolo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caula",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Vitali",
"given": "Emanuela",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Liuzzi",
"given": "Nicola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pastorelli",
"given": "Mauro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Caironi",
"given": "Pietro",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gamna",
"given": "Federica",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Scapino",
"given": "Bruno",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gurioli",
"given": "Lorenzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Magro",
"given": "Emanuele",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Roberti",
"given": "Giuseppe",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Santamaria",
"given": "Gian Mario",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Daffonchio",
"given": "Antonella",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Varese",
"given": "Paola",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ghiazza",
"given": "Gianfranco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Girino",
"given": "Margherita",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Pelazza",
"given": "Carolina",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Racca",
"given": "Fabrizio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Grillo",
"given": "Mirco",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Del Bono",
"given": "Valerio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gianotto",
"given": "Giorgio",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Aluffi",
"given": "Enzo",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ravera",
"given": "Enrico",
"sequence": "additional"
}
],
"clinical-trial-number": [
{
"clinical-trial-number": "nct04428021",
"registry": "10.18810/clinical-trials-gov"
}
],
"container-title": "BMC Infectious Diseases",
"container-title-short": "BMC Infect Dis",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2022,
11,
23
]
],
"date-time": "2022-11-23T09:29:15Z",
"timestamp": 1669195755000
},
"deposited": {
"date-parts": [
[
2022,
11,
23
]
],
"date-time": "2022-11-23T09:29:31Z",
"timestamp": 1669195771000
},
"indexed": {
"date-parts": [
[
2024,
8,
14
]
],
"date-time": "2024-08-14T20:31:19Z",
"timestamp": 1723667479385
},
"is-referenced-by-count": 4,
"issue": "1",
"issued": {
"date-parts": [
[
2022,
11,
22
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2022,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
22
]
],
"date-time": "2022-11-22T00:00:00Z",
"timestamp": 1669075200000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2022,
11,
22
]
],
"date-time": "2022-11-22T00:00:00Z",
"timestamp": 1669075200000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07716-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12879-022-07716-5/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12879-022-07716-5.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2022,
11,
22
]
]
},
"published-online": {
"date-parts": [
[
2022,
11,
22
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/S0140-6736(79)92335-3",
"author": "JI Maiztegui",
"doi-asserted-by": "publisher",
"first-page": "1216",
"journal-title": "Lancet Lond Engl",
"key": "7716_CR1",
"unstructured": "Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet Lond Engl. 1979;2:1216–7.",
"volume": "2",
"year": "1979"
},
{
"DOI": "10.3851/IMP3243",
"author": "J-H Ko",
"doi-asserted-by": "publisher",
"first-page": "617",
"journal-title": "Antivir Ther",
"key": "7716_CR2",
"unstructured": "Ko J-H, Seok H, Cho SY, Eun Ha Y, Baek JY, Kim SH, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. 2018;23:617–22.",
"volume": "23",
"year": "2018"
},
{
"DOI": "10.1007/s10096-004-1271-9",
"author": "Y Cheng",
"doi-asserted-by": "publisher",
"first-page": "44",
"journal-title": "Eur J Clin Microbiol Infect Dis",
"key": "7716_CR3",
"unstructured": "Cheng Y, Wong R, Soo YOY, Wong WS, Lee CK, Ng MHL, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–6.",
"volume": "24",
"year": "2005"
},
{
"DOI": "10.1093/cid/ciq106",
"author": "IF Hung",
"doi-asserted-by": "publisher",
"first-page": "447",
"journal-title": "Clin Infect Dis",
"key": "7716_CR4",
"unstructured": "Hung IF, To KK, Lee C-K, Lee K-L, Chan K, Yan W-W, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–56.",
"volume": "52",
"year": "2011"
},
{
"DOI": "10.1056/NEJMc070359",
"author": "B Zhou",
"doi-asserted-by": "publisher",
"first-page": "1450",
"journal-title": "N Engl J Med",
"key": "7716_CR5",
"unstructured": "Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1.",
"volume": "357",
"year": "2007"
},
{
"DOI": "10.1001/jama.2020.4783",
"author": "C Shen",
"doi-asserted-by": "publisher",
"first-page": "1582",
"journal-title": "JAMA",
"key": "7716_CR6",
"unstructured": "Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with covid-19 with convalescent plasma. JAMA. 2020;323:1582–9.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1073/pnas.2004168117",
"author": "K Duan",
"doi-asserted-by": "publisher",
"first-page": "9490",
"journal-title": "Proc Natl Acad Sci",
"key": "7716_CR7",
"unstructured": "Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci. 2020;117:9490–6.",
"volume": "117",
"year": "2020"
},
{
"DOI": "10.1101/2020.08.12.20169359",
"doi-asserted-by": "crossref",
"key": "7716_CR8",
"unstructured": "Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1016/j.transci.2020.102875",
"author": "H Abolghasemi",
"doi-asserted-by": "publisher",
"journal-title": "Transfus Apher Sci",
"key": "7716_CR9",
"unstructured": "Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfus Apher Sci. 2020;59.",
"volume": "59",
"year": "2020"
},
{
"DOI": "10.1182/blood.2020006964",
"author": "L Hegerova",
"doi-asserted-by": "publisher",
"first-page": "759",
"journal-title": "Blood",
"key": "7716_CR10",
"unstructured": "Hegerova L, Gooley TA, Sweerus KA, Maree C, Bailey N, Bailey M, et al. Use of convalescent plasma in hospitalized patients with COVID-19: case series. Blood. 2020;136:759–62.",
"volume": "136",
"year": "2020"
},
{
"DOI": "10.1101/2020.05.26.20113373",
"doi-asserted-by": "crossref",
"key": "7716_CR11",
"unstructured": "Perotti C, Baldanti F, Bruno R, Del Fante C, Seminari E, Casari S, et al. Mortality reduction in 46 severe COVID-19 patients treated with hyperimmune plasma. A proof of concept single arm multicenter interventional trial. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1101/2020.05.20.20102236",
"doi-asserted-by": "crossref",
"key": "7716_CR12",
"unstructured": "Liu STH, Lin H-M, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: a matched control study. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1016/j.ajpath.2020.08.001",
"author": "E Salazar",
"doi-asserted-by": "publisher",
"first-page": "2290",
"journal-title": "Am J Pathol",
"key": "7716_CR13",
"unstructured": "Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190:2290–303.",
"volume": "190",
"year": "2020"
},
{
"DOI": "10.1186/s12879-021-06829-7",
"author": "C Axfors",
"doi-asserted-by": "publisher",
"first-page": "1170",
"journal-title": "BMC Infect Dis",
"key": "7716_CR14",
"unstructured": "Axfors C, Janiaud P, Schmitt AM, van’t Hooft J, Smith ER, Haber NA, et al. Association between convalescent plasma treatment and mortality in COVID-19: a collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect Dis. 2021;21:1170.",
"volume": "21",
"year": "2021"
},
{
"DOI": "10.1093/ije/dyaa026",
"author": "A Odutayo",
"doi-asserted-by": "publisher",
"first-page": "968",
"journal-title": "Int J Epidemiol",
"key": "7716_CR15",
"unstructured": "Odutayo A, Gryaznov D, Copsey B, Monk P, Speich B, Roberts C, et al. Design, analysis and reporting of multi-arm trials and strategies to address multiple testing. Int J Epidemiol. 2020;49:968–78.",
"volume": "49",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.10044",
"author": "L Li",
"doi-asserted-by": "publisher",
"first-page": "460",
"journal-title": "JAMA",
"key": "7716_CR16",
"unstructured": "Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. 2020;324:460.",
"volume": "324",
"year": "2020"
},
{
"DOI": "10.1101/2020.07.01.20139857",
"doi-asserted-by": "crossref",
"key": "7716_CR17",
"unstructured": "Gharbharan A, Jordans CCE, Geurtsvankessel C, den Hollander JG, Karim F, Mollema FPN, et al. Convalescent Plasma for COVID-19. A randomized clinical trial. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1136/bmj.m3939",
"doi-asserted-by": "crossref",
"key": "7716_CR18",
"unstructured": "Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;m3939."
},
{
"DOI": "10.1056/NEJMoa2031304",
"author": "VA Simonovich",
"doi-asserted-by": "publisher",
"first-page": "619",
"journal-title": "N Engl J Med",
"key": "7716_CR19",
"unstructured": "Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021;384:619–29.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1101/2020.10.25.20219337",
"doi-asserted-by": "crossref",
"key": "7716_CR20",
"unstructured": "Bajpai M, Kumar S, Maheshwari A, Chhabra K, kale P, Gupta A, et al. Efficacy of convalescent plasma therapy compared to fresh frozen plasma in severely ill COVID-19 patients: a pilot randomized controlled trial. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1101/2020.11.02.20224303",
"doi-asserted-by": "crossref",
"key": "7716_CR21",
"unstructured": "AlQahtani M, Abdulrahman A, Almadani A, Alali SY, Al Zamrooni AM, Hejab AH, et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1371/journal.pmed.1003415",
"author": "ME Balcells",
"doi-asserted-by": "publisher",
"journal-title": "PLoS Med",
"key": "7716_CR22",
"unstructured": "Balcells ME, Rojas L, Le Corre N, Martínez-Valdebenito C, Ceballos ME, Ferrés M, et al. Early versus deferred anti-SARS-CoV-2 convalescent plasma in patients admitted for COVID-19: a randomized phase II clinical trial. PLoS Med. 2021;18.",
"volume": "18",
"year": "2021"
},
{
"DOI": "10.1101/2020.11.25.20237883",
"doi-asserted-by": "crossref",
"key": "7716_CR23",
"unstructured": "Ray Y, Paul SR, Bandopadhyay P, D’Rozario R, Sarif J, Lahiri A, et al. Clinical and immunological benefits of convalescent plasma therapy in severe COVID-19: insights from a single center open label randomised control trial. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1101/2020.08.26.20182444",
"doi-asserted-by": "crossref",
"key": "7716_CR24",
"unstructured": "Avendaño-Solà C, Ramos-Martínez A, Muñez-Rubio E, Ruiz-Antorán B, de Molina RM, Torres F, et al. Convalescent plasma for COVID-19: a multicenter, randomized clinical trial. Infect Dis (except HIV/AIDS); 2020."
},
{
"DOI": "10.1056/NEJMoa2033700",
"author": "R Libster",
"doi-asserted-by": "publisher",
"first-page": "610",
"journal-title": "N Engl J Med",
"key": "7716_CR25",
"unstructured": "Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–8.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1101/2021.03.12.21253373",
"doi-asserted-by": "crossref",
"key": "7716_CR26",
"unstructured": "O’Donnell MR, Grinsztejn B, Cummings MJ, Justman J, Lamb MR, Eckhardt CM, et al. A randomized, double-blind, controlled trial of convalescent plasma in adults with severe COVID-19. Infect Dis (except HIV/AIDS); 2021."
},
{
"DOI": "10.1016/S0140-6736(21)00897-7",
"author": "RECOVERY Collaborative Group",
"doi-asserted-by": "publisher",
"first-page": "2049",
"journal-title": "Lancet Lond Engl",
"key": "7716_CR27",
"unstructured": "RECOVERY Collaborative Group. Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): a randomised controlled, open-label, platform trial. Lancet Lond Engl. 2021;397:2049–59.",
"volume": "397",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2031893",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1015",
"journal-title": "N Engl J Med",
"key": "7716_CR28",
"unstructured": "Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from COVID-19. N Engl J Med. 2021;384:1015–27.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1038/s41591-021-01488-2",
"author": "P Bégin",
"doi-asserted-by": "publisher",
"journal-title": "Nat Med",
"key": "7716_CR29",
"unstructured": "Bégin P, Callum J, Jamula E, Cook R, Heddle NM, Tinmouth A, et al. Convalescent plasma for hospitalized patients with COVID-19: an open-label, randomized controlled trial. Nat Med. 2021. https://doi.org/10.1038/s41591-021-01488-2.",
"year": "2021"
},
{
"DOI": "10.1001/jamanetworkopen.2021.36246",
"author": "F Menichetti",
"doi-asserted-by": "publisher",
"journal-title": "JAMA Netw Open",
"key": "7716_CR30",
"unstructured": "Menichetti F, Popoli P, Puopolo M, Spila Alegiani S, Tiseo G, Bartoloni A, et al. Effect of high-titer convalescent plasma on progression to severe respiratory failure or death in hospitalized patients with COVID-19 pneumonia: a randomized clinical trial. JAMA Netw Open. 2021;4.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2103784",
"author": "FK Korley",
"doi-asserted-by": "publisher",
"first-page": "1951",
"journal-title": "N Engl J Med",
"key": "7716_CR31",
"unstructured": "Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, et al. Early convalescent plasma for high-risk outpatients with COVID-19. N Engl J Med. 2021;385:1951.",
"volume": "385",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa449",
"author": "X Chen",
"doi-asserted-by": "publisher",
"first-page": "1937",
"journal-title": "Clin Infect Dis",
"key": "7716_CR32",
"unstructured": "Chen X, Zhao B, Qu Y, Chen Y, Xiong J, Feng Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71:1937–42.",
"volume": "71",
"year": "2020"
},
{
"DOI": "10.1080/22221751.2020.1732837",
"author": "W Chen",
"doi-asserted-by": "publisher",
"first-page": "469",
"journal-title": "Emerg Microbes Infect",
"key": "7716_CR33",
"unstructured": "Chen W, Lan Y, Yuan X, Deng X, Li Y, Cai X, et al. Detectable 2019-nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerg Microbes Infect. 2020;9:469–73.",
"volume": "9",
"year": "2020"
},
{
"DOI": "10.1093/cid/ciaa1054",
"author": "CA Hogan",
"doi-asserted-by": "publisher",
"first-page": "e291",
"journal-title": "Clin Infect Dis",
"key": "7716_CR34",
"unstructured": "Hogan CA, Stevens BA, Sahoo MK, Huang C, Garamani N, Gombar S, et al. High frequency of SARS-CoV-2 RNAemia and association with severe disease. Clin Infect Dis. 2021;72:e291–5.",
"volume": "72",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1285",
"author": "K Hagman",
"doi-asserted-by": "publisher",
"first-page": "e2995",
"journal-title": "Clin Infect Dis",
"key": "7716_CR35",
"unstructured": "Hagman K, Hedenstierna M, Gille-Johnson P, Hammas B, Grabbe M, Dillner J, et al. Severe acute respiratory syndrome coronavirus 2 RNA in serum as predictor of severe outcome in coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis. 2020;73:e2995.",
"volume": "73",
"year": "2020"
},
{
"DOI": "10.3390/v12091045",
"author": "KA Eberhardt",
"doi-asserted-by": "publisher",
"first-page": "1045",
"journal-title": "Viruses",
"key": "7716_CR36",
"unstructured": "Eberhardt KA, Meyer-Schwickerath C, Heger E, Knops E, Lehmann C, Rybniker J, et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID-19 patients. Viruses. 2020;12:1045.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1016/j.mayocp.2020.06.028",
"author": "MJ Joyner",
"doi-asserted-by": "publisher",
"first-page": "1888",
"journal-title": "Mayo Clin Proc",
"key": "7716_CR37",
"unstructured": "Joyner MJ, Bruno KA, Klassen SA, Kunze KL, Johnson PW, Lesser ER, et al. Safety update: COVID-19 convalescent plasma in 20,000 hospitalized patients. Mayo Clin Proc. 2020;95:1888–97.",
"volume": "95",
"year": "2020"
},
{
"DOI": "10.1136/bmj.m4072",
"doi-asserted-by": "crossref",
"key": "7716_CR38",
"unstructured": "Pathak EB. Convalescent plasma is ineffective for covid-19. BMJ. 2020;m4072."
}
],
"reference-count": 38,
"references-count": 38,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-022-07716-5"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [],
"subtitle": [],
"title": "Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: short and long-term effects. A three-arm randomized controlled clinical trial",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "22"
}