Clinical characteristics and outcomes among critically ill patients with cancer and COVID-19-related acute respiratory failure
et al., BMC Pulmonary Medicine, doi:10.1186/s12890-024-02850-z, Jan 2024
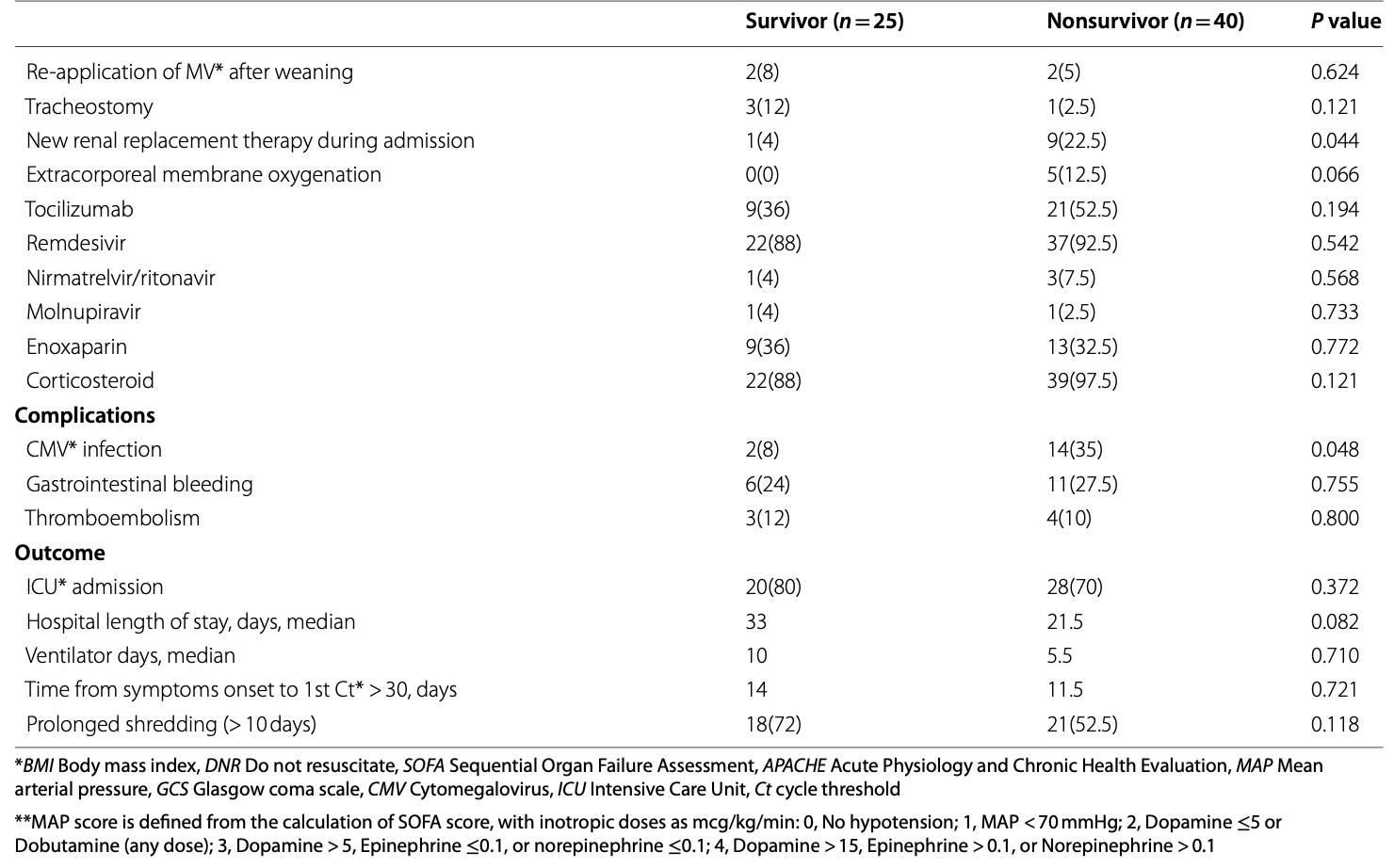
Retrospective study of 215 critically ill COVID-19 patients with respiratory failure showing higher mortality for cancer patients. Remdesivir was used more for non-survivors, without statistical significance. Most patients received remdesivir, suggesting standard use for critically ill patients at the time, however it is not clear why some patients did not receive treatment, and baseline details per group are not provided.
Gérard, Zhou, Wu, Kamo, Choi, Kim show increased risk of acute kidney injury, Leo, Briciu, Muntean, Petrov show increased risk of liver injury, and Negru, Cheng, Mohammed, Kwok show increased risk of cardiac disorders with remdesivir.
This study is excluded in the after exclusion results of meta-analysis:
unadjusted results with no group details.
|
risk of death, 25.4% higher, RR 1.25, p = 0.67, treatment 37 of 59 (62.7%), control 3 of 6 (50.0%), day 120.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Gérard et al., Remdesivir and Acute Renal Failure: A Potential Safety Signal From Disproportionality Analysis of the WHO Safety Database, Clinical Pharmacology & Therapeutics, doi:10.1002/cpt.2145.
2.
Zhou et al., Acute Kidney Injury and Drugs Prescribed for COVID-19 in Diabetes Patients: A Real-World Disproportionality Analysis, Frontiers in Pharmacology, doi:10.3389/fphar.2022.833679.
3.
Wu et al., Acute Kidney Injury Associated With Remdesivir: A Comprehensive Pharmacovigilance Analysis of COVID-19 Reports in FAERS, Frontiers in Pharmacology, doi:10.3389/fphar.2022.692828.
4.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
5.
Choi et al., Comparative effectiveness of combination therapy with nirmatrelvir–ritonavir and remdesivir versus monotherapy with remdesivir or nirmatrelvir–ritonavir in patients hospitalised with COVID-19: a target trial emulation study, The Lancet Infectious Diseases, doi:10.1016/S1473-3099(24)00353-0.
6.
Kim et al., Investigating the Safety Profile of Fast‐Track COVID‐19 Drugs Using the FDA Adverse Event Reporting System Database: A Comparative Observational Study, Pharmacoepidemiology and Drug Safety, doi:10.1002/pds.70043.
7.
Leo et al., Hepatocellular liver injury in hospitalized patients affected by COVID-19: Presence of different risk factors at different time points, Digestive and Liver Disease, doi:10.1016/j.dld.2021.12.014.
8.
Briciu et al., Evolving Clinical Manifestations and Outcomes in COVID-19 Patients: A Comparative Analysis of SARS-CoV-2 Variant Waves in a Romanian Hospital Setting, Pathogens, doi:10.3390/pathogens12121453.
9.
Muntean et al., Effects of COVID-19 on the Liver and Mortality in Patients with SARS-CoV-2 Pneumonia Caused by Delta and Non-Delta Variants: An Analysis in a Single Centre, Pharmaceuticals, doi:10.3390/ph17010003.
10.
Petrov et al., The Effect of Potentially Hepatotoxic Medicinal Products on Alanine Transaminase Levels in COVID-19 Patients: A Case–Control Study, Safety and Risk of Pharmacotherapy, doi:10.30895/2312-7821-2025-458.
11.
Negru et al., Comparative Pharmacovigilance Analysis of Approved and Repurposed Antivirals for COVID-19: Insights from EudraVigilance Data, Biomedicines, doi:10.3390/biomedicines13061387.
12.
Cheng et al., Cardiovascular Safety of COVID-19 Treatments: A Disproportionality Analysis of Adverse Event Reports from the WHO VigiBase, Infectious Diseases and Therapy, doi:10.1007/s40121-025-01225-z.
Liao et al., 15 Jan 2024, retrospective, Taiwan, peer-reviewed, median age 73.0, 10 authors, study period May 2022 - September 2022.
Contact: wiji.chen@gmail.com.
Clinical characteristics and outcomes among critically ill patients with cancer and COVID-19-related acute respiratory failure
BMC Pulmonary Medicine, doi:10.1186/s12890-024-02850-z
Background Coronavirus disease 2019 (COVID-19) has affected individuals worldwide, and patients with cancer are particularly vulnerable to COVID-19-related severe illness, respiratory failure, and mortality. The relationship between COVID-19 and cancer remains a critical concern, and a comprehensive investigation of the factors affecting survival among patients with cancer who develop COVID-19-related respiratory failure is warranted. We aim to compare the characteristics and outcomes of COVID-19-related acute respiratory failure in patients with and without underlying cancer, while analyzing factors affecting in-hospital survival among cancer patients.
Methods We conducted a retrospective observational study at Taipei Veterans General Hospital in Taiwan from May to September 2022, a period during which the omicron variant of the severe acute respiratory syndrome coronavirus 2 was circulating. Eligible patients had COVID-19 and acute respiratory failure. Clinical data, demographic information, disease severity markers, treatment details, and outcomes were collected and analyzed.
Results Of the 215 enrolled critically ill patients with COVID-19, 65 had cancer. The patients with cancer were younger and had lower absolute lymphocyte counts, higher ferritin and lactate dehydrogenase (LDH) concentrations, and increased vasopressor use compared with those without cancer. The patients with cancer also received more COVID-19 specific treatments but had higher in-hospital mortality rate (61.5% vs 36%, P = 0.002) and longer viral shedding (13 vs 10 days, P = 0.007) than those without cancer did. Smoking [odds ratio (OR): 5.804, 95% confidence interval (CI): 1.847-39.746], elevated LDH (OR: 1.004, 95% CI: 1.001-1.012), vasopressor use (OR: 5.437, 95% CI: 1.202-24.593), and new renal replacement therapy (OR: 3.523, 95% CI: 1.203-61.108) were independent predictors of in-hospital mortality among patients with cancer and respiratory failure.
Conclusion Critically ill patients with cancer experiencing COVID-19-related acute respiratory failure present unique clinical features and worse clinical outcomes compared with those without cancer. Smoking, elevated LDH, vasopressor use, and new renal replacement therapy were risk factors for in-hospital mortality in these patients.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s12890-024-02850-z. Additional file 1.
Authors' contributions Conceptualization: Ying-Ting Liao, Hsiao-Chin Shen, Jhong-Ru Huang, Chuan-Yen Sun, Hung-Jui Ko, Chih-Jung Chang, Jia-Yih Feng, Wei-Chih Chen, Kuang-Yao Yang. Supervision: Wei-Chih Chen, Jia-Yih Feng, Kuang-Yao Yang, Yuh-Min Chen. Data Collection and/or Processing: Hsiao-Chin Shen, Chuan-Yen Sun, Jhong-Ru Huang, Ying-Ting Liao, Hung-Jui Ko, Chih-Jung Chang. Analysis and/ or Interpretation: Ying-Ting Liao, Wei-Chih Chen, Kuang-Yao Yang. Writingoriginal draft: Ying-Ting Liao, Wei-Chih Chen, Kuang-Yao Yang. Writing -review and editing: Ying-Ting Liao, Wei-Chih Chen, Kuang-Yao Yang. All authors read and approved the final manuscript.
Declarations Ethics approval and consent to participate This retrospective study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Ethical Review Board of Taipei Veterans General Hospital (Approval No. 2022-11-002 AC). Written informed consent was waived by Institutional Ethical Review Board of Taipei Veterans General Hospital due to retrospective design of the study.
Consent for publication Not applicable.
Competing interests The authors declare no competing interests.
Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Aboueshia, Hussein, Attia, Cancer and COVID-19: analysis of patient outcomes, Future Oncol, doi:10.2217/fon-2021-0121
Alhazzani, Møller, Arabi, Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19), Intensive Care Med, doi:10.1007/s00134-020-06022-5
Aziz, Haghbin, Sitta, Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis, J Med Virol, doi:10.1002/jmv.26509
Berlin, Gulick, Martinez, Severe Covid-19, N Engl J Med, doi:10.1056/NEJMcp2009575
Cai, Gao, Zeng, Immunological alternation in COVID-19 patients with cancer and its implications on mortality, Oncoimmunology, doi:10.1080/2162402x.2020.1854424
Coomes, Haghbayan, Interleukin-6 in Covid-19: a systematic review and meta-analysis, Rev Med Virol, doi:10.1002/rmv.2141
Elhadad, Bronstein, Yana, Characteristics and outcomes of patients infected with SARS-CoV-2 in Israel: correlation between laboratory findings on admission to emergency department and subsequent respiratory failure, Isr Med Assoc J
Espejo-Paeres, Núñez-Gil, Estrada, Impact of smoking on COVID-19 outcomes: a HOPE registry subanalysis, BMJ Nutr Prev Health, doi:10.1136/bmjnph-2021-000269
Feld, Tremblay, Thibaud, Kessler, Naymagon, Ferritin levels in patients with COVID-19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis, Int J Lab Hematol, doi:10.1111/ijlh.13309
Garassino, Whisenant, Huang, COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study, The Lancet Oncol, doi:10.1016/S1470-2045(20)30314-4
Goubet, Dubuisson, Geraud, Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis, Cell Death Different, doi:10.1038/s41418-021-00817-9
Grivas, Khaki, Wise-Draper, Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer consortium, Ann Oncol, doi:10.1016/j.annonc.2021.02.024
Gómez-Pastora, Weigand, Kim, Hyperferritinemia in critically ill COVID-19 patients -is ferritin the product of inflammation or a pathogenic mediator?, Clin Chim Acta, doi:10.1016/j.cca.2020.06.033
Hauser, Tabak, Baier, Survival of patients with Cancer in a medical critical care unit, Arch Intern Med, doi:10.1001/archinte.1982.00340160107022
Hirano, IL-6 in inflammation, autoimmunity and cancer, Int Immunol, doi:10.1093/intimm/dxaa078
Horby, Lim, Emberson, Dexamethasone in Hospitalized Patients with Covid-19, N Engl J Med, doi:10.1056/NEJMoa2021436
Illg, Muller, Mueller, Nippert, Allen, Analysis of absolute lymphocyte count in patients with COVID-19, Am J Emerg Med, doi:10.1016/j.ajem.2021.02.054
Jee, Foote, Lumish, Chemotherapy and COVID-19 outcomes in patients with Cancer, J Clin Oncol, doi:10.1200/jco.20.01307
Kao, Lai, Hung, Sequential oxygenation index and organ dysfunction assessment within the first 3 days of mechanical ventilation predict the outcome of adult patients with severe acute respiratory failure, Sci World J, doi:10.1155/2013/413216
Kaushal, Kaur, Sarma, Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis, J Crit Care, doi:10.1016/j.jcrc.2021.09.023
Knaus, Draper, Wagner, Zimmerman, APACHE II: a severity of disease classification system, Crit Care Med
Kumari, Dwarakanath, Das, Bhatt, Role of interleukin-6 in cancer progression and therapeutic resistance, Tumour Biol, doi:10.1007/s13277-016-5098-7
Lecuyer, Chevret, Thiery, Darmon, Schlemmer et al., The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation, Crit Care Med, doi:10.1097/01.Ccm.0000256846.27192.7a
Lee, Cazier, Angelis, COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study, Lancet, doi:10.1016/s0140-6736(20)31173-9
Li, Ye, Chen, Elevated Lactate Dehydrogenase (LDH) level as an independent risk factor for the severity and mortality of COVID-19, Aging, doi:10.18632/aging.103770
Lippi, Henry, Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19), Eur J Intern Med, doi:10.1016/j.ejim.2020.03.014
Malik, Patel, Mehta, Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis, BMJ Evid Based Med, doi:10.1136/bmjebm-2020-111536
Mehta, Goel, Kabarriti, Case fatality rate of Cancer patients with COVID-19 in a New York hospital system, Cancer Discov, doi:10.1158/2159-8290.Cd-20-0516
Mesa, César, Martín-Montañez, Acute lung injury biomarkers in the prediction of COVID-19 severity: Total thiol, ferritin and lactate dehydrogenase, Antioxidants, doi:10.3390/antiox10081221
Michard, Malbrain, Martin, Haemodynamic monitoring and management in COVID-19 intensive care patients: an international survey, Anaesth Crit Care Pain Med, doi:10.1016/j.accpm.2020.08.001
Patanavanich, Glantz, Smoking Is Associated With COVID-19 Progression: A Meta-analysis, Nicotine Tob Res, doi:10.1093/ntr/ntaa082
Salama, Han, Yau, Tocilizumab in patients hospitalized with Covid-19 pneumonia, N Engl J Med, doi:10.1056/NEJMoa2030340
Smith, Sausville, Girish, Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract, Dev Cell, doi:10.1016/j.devcel.2020.05.012
Szarpak, Ruetzler, Safiejko, Lactate dehydrogenase level as a COVID-19 severity marker, Am J Emerg Med, doi:10.1016/j.ajem.2020.11.025
Teasdale, Jennett, Assessment of coma and impaired consciousness. A practical scale, Lancet, doi:10.1016/s0140-6736(74)91639-0
Topp, Bouyea, Cochran-Caggiano, Biomarkers predictive of Extubation and survival of COVID-19 patients, Cureus, doi:10.7759/cureus.15462
Van Paassen, Vos, Hoekstra, Neumann, Boot et al., Corticosteroid use in COVID-19 patients: a systematic review and metaanalysis on clinical outcomes, Crit Care, doi:10.1186/s13054-020-03400-9
Vincent, Moreno, Takala, The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine, Intensive Care Med, doi:10.1007/bf01709751
Yekedüz, Utkan, Ürün, A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19, Eur J Cancer, doi:10.1016/j.ejca.2020.09.028
Young, Ong, Ng, Viral Dynamics and Immune Correlates of Coronavirus Disease 2019 (COVID-19) Severity, Clin Infect Dis, doi:10.1093/cid/ciaa1280
Zhang, Zhong, Wu, Cancer treatment in the coronavirus disease pandemic, Lung Cancer, doi:10.1016/j.lungcan.2020.12.012
Zhao, Meng, Kumar, The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis, J Med Virol, doi:10.1002/jmv.25889
Zong, Wei, Ren, Zhang, Zhou, The intersection of COVID-19 and cancer: signaling pathways and treatment implications, Mol Cancer, doi:10.1186/s12943-021-01363-1
DOI record:
{
"DOI": "10.1186/s12890-024-02850-z",
"ISSN": [
"1471-2466"
],
"URL": "http://dx.doi.org/10.1186/s12890-024-02850-z",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>Coronavirus disease 2019 (COVID-19) has affected individuals worldwide, and patients with cancer are particularly vulnerable to COVID-19-related severe illness, respiratory failure, and mortality. The relationship between COVID-19 and cancer remains a critical concern, and a comprehensive investigation of the factors affecting survival among patients with cancer who develop COVID-19-related respiratory failure is warranted. We aim to compare the characteristics and outcomes of COVID-19-related acute respiratory failure in patients with and without underlying cancer, while analyzing factors affecting in-hospital survival among cancer patients.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>We conducted a retrospective observational study at Taipei Veterans General Hospital in Taiwan from May to September 2022, a period during which the omicron variant of the severe acute respiratory syndrome coronavirus 2 was circulating. Eligible patients had COVID-19 and acute respiratory failure. Clinical data, demographic information, disease severity markers, treatment details, and outcomes were collected and analyzed.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Of the 215 enrolled critically ill patients with COVID-19, 65 had cancer. The patients with cancer were younger and had lower absolute lymphocyte counts, higher ferritin and lactate dehydrogenase (LDH) concentrations, and increased vasopressor use compared with those without cancer. The patients with cancer also received more COVID-19 specific treatments but had higher in-hospital mortality rate (61.5% vs 36%, <jats:italic>P =</jats:italic> 0.002) and longer viral shedding (13 vs 10 days, <jats:italic>P =</jats:italic> 0.007) than those without cancer did. Smoking [odds ratio (OR): 5.804, 95% confidence interval (CI): 1.847–39.746], elevated LDH (OR: 1.004, 95% CI: 1.001–1.012), vasopressor use (OR: 5.437, 95% CI: 1.202–24.593), and new renal replacement therapy (OR: 3.523, 95% CI: 1.203–61.108) were independent predictors of in-hospital mortality among patients with cancer and respiratory failure.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusion</jats:title>\n <jats:p>Critically ill patients with cancer experiencing COVID-19-related acute respiratory failure present unique clinical features and worse clinical outcomes compared with those without cancer. Smoking, elevated LDH, vasopressor use, and new renal replacement therapy were risk factors for in-hospital mortality in these patients.</jats:p>\n </jats:sec>",
"alternative-id": [
"2850"
],
"article-number": "34",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "18 September 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "5 January 2024"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "15 January 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "This retrospective study was performed in accordance with the Declaration of Helsinki and approved by the Institutional Ethical Review Board of Taipei Veterans General Hospital (Approval No. 2022–11-002 AC). Written informed consent was waived by Institutional Ethical Review Board of Taipei Veterans General Hospital due to retrospective design of the study."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "The authors declare no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Liao",
"given": "Ying-Ting",
"sequence": "first"
},
{
"affiliation": [],
"family": "Shen",
"given": "Hsiao-Chin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huang",
"given": "Jhong-Ru",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sun",
"given": "Chuan-Yen",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ko",
"given": "Hung-Jui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chang",
"given": "Chih-Jung",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Yuh-Min",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Feng",
"given": "Jia-Yih",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Wei-Chih",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Yang",
"given": "Kuang-Yao",
"sequence": "additional"
}
],
"container-title": "BMC Pulmonary Medicine",
"container-title-short": "BMC Pulm Med",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T08:02:34Z",
"timestamp": 1705305754000
},
"deposited": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T19:02:33Z",
"timestamp": 1705345353000
},
"indexed": {
"date-parts": [
[
2024,
1,
16
]
],
"date-time": "2024-01-16T00:13:46Z",
"timestamp": 1705364026834
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
15
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T00:00:00Z",
"timestamp": 1705276800000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
15
]
],
"date-time": "2024-01-15T00:00:00Z",
"timestamp": 1705276800000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12890-024-02850-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s12890-024-02850-z/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s12890-024-02850-z.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
1,
15
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
15
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"key": "2850_CR1",
"unstructured": "WHO Coronavirus (COVID-19) Dashboard. Accessed Nov 27, 2023. https://covid19.who.int/"
},
{
"DOI": "10.1016/j.lungcan.2020.12.012",
"author": "JT Zhang",
"doi-asserted-by": "publisher",
"first-page": "98",
"journal-title": "Lung Cancer.",
"key": "2850_CR2",
"unstructured": "Zhang JT, Zhong WZ, Wu YL. Cancer treatment in the coronavirus disease pandemic. Lung Cancer. 2021;152:98–103. https://doi.org/10.1016/j.lungcan.2020.12.012.",
"volume": "152",
"year": "2021"
},
{
"DOI": "10.1158/2159-8290.Cd-20-0516",
"author": "V Mehta",
"doi-asserted-by": "publisher",
"first-page": "935",
"issue": "7",
"journal-title": "Cancer Discov.",
"key": "2850_CR3",
"unstructured": "Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of Cancer patients with COVID-19 in a New York hospital system. Cancer Discov. 2020;10(7):935–41. https://doi.org/10.1158/2159-8290.Cd-20-0516.",
"volume": "10",
"year": "2020"
},
{
"DOI": "10.1056/NEJMcp2009575",
"author": "DA Berlin",
"doi-asserted-by": "publisher",
"first-page": "2451",
"issue": "25",
"journal-title": "N Engl J Med.",
"key": "2850_CR4",
"unstructured": "Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–60. https://doi.org/10.1056/NEJMcp2009575.",
"volume": "383",
"year": "2020"
},
{
"DOI": "10.1007/s00134-020-06022-5",
"author": "W Alhazzani",
"doi-asserted-by": "publisher",
"first-page": "854",
"issue": "5",
"journal-title": "Intensive Care Med.",
"key": "2850_CR5",
"unstructured": "Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854–87. https://doi.org/10.1007/s00134-020-06022-5.",
"volume": "46",
"year": "2020"
},
{
"DOI": "10.1056/NEJMoa2021436",
"author": "P Horby",
"doi-asserted-by": "publisher",
"first-page": "693",
"issue": "8",
"journal-title": "N Engl J Med.",
"key": "2850_CR6",
"unstructured": "Horby P, Lim WS, Emberson JR, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. https://doi.org/10.1056/NEJMoa2021436.",
"volume": "384",
"year": "2021"
},
{
"DOI": "10.1016/s0140-6736(20)31173-9",
"author": "LY Lee",
"doi-asserted-by": "publisher",
"first-page": "1919",
"issue": "10241",
"journal-title": "Lancet.",
"key": "2850_CR7",
"unstructured": "Lee LY, Cazier JB, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395(10241):1919–26. https://doi.org/10.1016/s0140-6736(20)31173-9.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1016/j.annonc.2021.02.024",
"author": "P Grivas",
"doi-asserted-by": "publisher",
"first-page": "787",
"issue": "6",
"journal-title": "Ann Oncol.",
"key": "2850_CR8",
"unstructured": "Grivas P, Khaki AR, Wise-Draper TM, et al. Association of clinical factors and recent anticancer therapy with COVID-19 severity among patients with cancer: a report from the COVID-19 and Cancer consortium. Ann Oncol. 2021;32(6):787–800. https://doi.org/10.1016/j.annonc.2021.02.024.",
"volume": "32",
"year": "2021"
},
{
"DOI": "10.2217/fon-2021-0121",
"author": "M Aboueshia",
"doi-asserted-by": "publisher",
"first-page": "3499",
"issue": "26",
"journal-title": "Future Oncol.",
"key": "2850_CR9",
"unstructured": "Aboueshia M, Hussein MH, Attia AS, et al. Cancer and COVID-19: analysis of patient outcomes. Future Oncol. 2021;17(26):3499–510. https://doi.org/10.2217/fon-2021-0121.",
"volume": "17",
"year": "2021"
},
{
"DOI": "10.1016/S1470-2045(20)30314-4",
"author": "MC Garassino",
"doi-asserted-by": "publisher",
"first-page": "914",
"issue": "7",
"journal-title": "The Lancet Oncol.",
"key": "2850_CR10",
"unstructured": "Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. The Lancet Oncol. 2020;21(7):914–22. https://doi.org/10.1016/S1470-2045(20)30314-4.",
"volume": "21",
"year": "2020"
},
{
"DOI": "10.1200/jco.20.01307",
"author": "J Jee",
"doi-asserted-by": "publisher",
"first-page": "3538",
"issue": "30",
"journal-title": "J Clin Oncol.",
"key": "2850_CR11",
"unstructured": "Jee J, Foote MB, Lumish M, et al. Chemotherapy and COVID-19 outcomes in patients with Cancer. J Clin Oncol. 2020;38(30):3538–46. https://doi.org/10.1200/jco.20.01307.",
"volume": "38",
"year": "2020"
},
{
"DOI": "10.1016/j.ejca.2020.09.028",
"author": "E Yekedüz",
"doi-asserted-by": "publisher",
"first-page": "92",
"journal-title": "Eur J Cancer.",
"key": "2850_CR12",
"unstructured": "Yekedüz E, Utkan G, Ürün Y. A systematic review and meta-analysis: the effect of active cancer treatment on severity of COVID-19. Eur J Cancer. 2020;141:92–104. https://doi.org/10.1016/j.ejca.2020.09.028.",
"volume": "141",
"year": "2020"
},
{
"DOI": "10.1007/bf01709751",
"author": "JL Vincent",
"doi-asserted-by": "publisher",
"first-page": "707",
"issue": "7",
"journal-title": "Intensive Care Med.",
"key": "2850_CR13",
"unstructured": "Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. https://doi.org/10.1007/bf01709751.",
"volume": "22",
"year": "1996"
},
{
"DOI": "10.1097/00003246-198510000-00009",
"author": "WA Knaus",
"doi-asserted-by": "publisher",
"first-page": "818",
"issue": "10",
"journal-title": "Crit Care Med.",
"key": "2850_CR14",
"unstructured": "Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29.",
"volume": "13",
"year": "1985"
},
{
"DOI": "10.1016/s0140-6736(74)91639-0",
"author": "G Teasdale",
"doi-asserted-by": "publisher",
"first-page": "81",
"issue": "7872",
"journal-title": "Lancet.",
"key": "2850_CR15",
"unstructured": "Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–4. https://doi.org/10.1016/s0140-6736(74)91639-0.",
"volume": "2",
"year": "1974"
},
{
"DOI": "10.1155/2013/413216",
"author": "HC Kao",
"doi-asserted-by": "publisher",
"first-page": "413216",
"journal-title": "Sci World J.",
"key": "2850_CR16",
"unstructured": "Kao HC, Lai TY, Hung HL, et al. Sequential oxygenation index and organ dysfunction assessment within the first 3 days of mechanical ventilation predict the outcome of adult patients with severe acute respiratory failure. Sci World J. 2013;2013:413216. https://doi.org/10.1155/2013/413216.",
"volume": "2013",
"year": "2013"
},
{
"DOI": "10.1080/2162402x.2020.1854424",
"author": "G Cai",
"doi-asserted-by": "publisher",
"first-page": "1854424",
"issue": "1",
"journal-title": "Oncoimmunology.",
"key": "2850_CR17",
"unstructured": "Cai G, Gao Y, Zeng S, et al. Immunological alternation in COVID-19 patients with cancer and its implications on mortality. Oncoimmunology. 2021;10(1):1854424. https://doi.org/10.1080/2162402x.2020.1854424.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1093/cid/ciaa1280",
"author": "BE Young",
"doi-asserted-by": "publisher",
"first-page": "e2932",
"issue": "9",
"journal-title": "Clin Infect Dis.",
"key": "2850_CR18",
"unstructured": "Young BE, Ong SWX, Ng LFP, et al. Viral Dynamics and Immune Correlates of Coronavirus Disease 2019 (COVID-19) Severity. Clin Infect Dis. 2021;73(9):e2932–42. https://doi.org/10.1093/cid/ciaa1280.",
"volume": "73",
"year": "2021"
},
{
"key": "2850_CR19",
"unstructured": "CECC revises criteria for releasing COVID-19 patients with moderate and severe symptoms from isolation to preserve isolation care capacity 2022. Accessed Nov 27, 2023. https://covid19.mohw.gov.tw/en/cp-4868-69897-206.html"
},
{
"DOI": "10.1016/j.ajem.2021.02.054",
"author": "Z Illg",
"doi-asserted-by": "publisher",
"first-page": "16",
"journal-title": "Am J Emerg Med.",
"key": "2850_CR20",
"unstructured": "Illg Z, Muller G, Mueller M, Nippert J, Allen B. Analysis of absolute lymphocyte count in patients with COVID-19. Am J Emerg Med. 2021;46:16–9. https://doi.org/10.1016/j.ajem.2021.02.054.",
"volume": "46",
"year": "2021"
},
{
"author": "D Elhadad",
"first-page": "605",
"issue": "10",
"journal-title": "Isr Med Assoc J.",
"key": "2850_CR21",
"unstructured": "Elhadad D, Bronstein Y, Yana M, et al. Characteristics and outcomes of patients infected with SARS-CoV-2 in Israel: correlation between laboratory findings on admission to emergency department and subsequent respiratory failure. Isr Med Assoc J. 2020;22(10):605–11.",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1136/bmjebm-2020-111536",
"author": "P Malik",
"doi-asserted-by": "publisher",
"first-page": "107",
"issue": "3",
"journal-title": "BMJ Evid Based Med.",
"key": "2850_CR22",
"unstructured": "Malik P, Patel U, Mehta D, et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26(3):107–8. https://doi.org/10.1136/bmjebm-2020-111536.",
"volume": "26",
"year": "2021"
},
{
"DOI": "10.1111/ijlh.13309",
"author": "J Feld",
"doi-asserted-by": "publisher",
"first-page": "773",
"issue": "6",
"journal-title": "Int J Lab Hematol.",
"key": "2850_CR23",
"unstructured": "Feld J, Tremblay D, Thibaud S, Kessler A, Naymagon L. Ferritin levels in patients with COVID-19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2020;42(6):773–9. https://doi.org/10.1111/ijlh.13309.",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1016/j.cca.2020.06.033",
"author": "J Gómez-Pastora",
"doi-asserted-by": "publisher",
"first-page": "249",
"journal-title": "Clin Chim Acta.",
"key": "2850_CR24",
"unstructured": "Gómez-Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID-19 patients - is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249–51. https://doi.org/10.1016/j.cca.2020.06.033.",
"volume": "509",
"year": "2020"
},
{
"DOI": "10.1016/j.jcrc.2021.09.023",
"author": "K Kaushal",
"doi-asserted-by": "publisher",
"first-page": "172",
"journal-title": "J Crit Care.",
"key": "2850_CR25",
"unstructured": "Kaushal K, Kaur H, Sarma P, et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care. Feb 2022;67:172–81. https://doi.org/10.1016/j.jcrc.2021.09.023.",
"volume": "67",
"year": "2022"
},
{
"DOI": "10.7759/cureus.15462",
"author": "G Topp",
"doi-asserted-by": "publisher",
"first-page": "e15462",
"issue": "6",
"journal-title": "Cureus.",
"key": "2850_CR26",
"unstructured": "Topp G, Bouyea M, Cochran-Caggiano N, et al. Biomarkers predictive of Extubation and survival of COVID-19 patients. Cureus. 2021;13(6):e15462. https://doi.org/10.7759/cureus.15462.",
"volume": "13",
"year": "2021"
},
{
"DOI": "10.3390/antiox10081221",
"doi-asserted-by": "publisher",
"key": "2850_CR27",
"unstructured": "Martinez Mesa A, Cabrera César E, Martín-Montañez E, et al. Acute lung injury biomarkers in the prediction of COVID-19 severity: Total thiol, ferritin and lactate dehydrogenase. Antioxidants (Basel). 2021;10(8) https://doi.org/10.3390/antiox10081221."
},
{
"DOI": "10.18632/aging.103770",
"author": "C Li",
"doi-asserted-by": "publisher",
"first-page": "15670",
"issue": "15",
"journal-title": "Aging (Albany NY).",
"key": "2850_CR28",
"unstructured": "Li C, Ye J, Chen Q, et al. Elevated Lactate Dehydrogenase (LDH) level as an independent risk factor for the severity and mortality of COVID-19. Aging (Albany NY). 2020;12(15):15670–81. https://doi.org/10.18632/aging.103770.",
"volume": "12",
"year": "2020"
},
{
"DOI": "10.1002/rmv.2141",
"author": "EA Coomes",
"doi-asserted-by": "publisher",
"first-page": "1",
"issue": "6",
"journal-title": "Rev Med Virol.",
"key": "2850_CR29",
"unstructured": "Coomes EA, Haghbayan H. Interleukin-6 in Covid-19: a systematic review and meta-analysis. Rev Med Virol. 2020;30(6):1–9. https://doi.org/10.1002/rmv.2141.",
"volume": "30",
"year": "2020"
},
{
"DOI": "10.1007/s13277-016-5098-7",
"author": "N Kumari",
"doi-asserted-by": "publisher",
"first-page": "11553",
"issue": "9",
"journal-title": "Tumour Biol.",
"key": "2850_CR30",
"unstructured": "Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553–72. https://doi.org/10.1007/s13277-016-5098-7.",
"volume": "37",
"year": "2016"
},
{
"DOI": "10.1093/intimm/dxaa078",
"author": "T Hirano",
"doi-asserted-by": "publisher",
"first-page": "127",
"issue": "3",
"journal-title": "Int Immunol.",
"key": "2850_CR31",
"unstructured": "Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021;33(3):127–48. https://doi.org/10.1093/intimm/dxaa078.",
"volume": "33",
"year": "2021"
},
{
"DOI": "10.1186/s13054-020-03400-9",
"author": "J van Paassen",
"doi-asserted-by": "publisher",
"first-page": "696",
"issue": "s",
"journal-title": "Crit Care.",
"key": "2850_CR32",
"unstructured": "van Paassen J, Vos JS, Hoekstra EM, Neumann KMI, Boot PC, Arbous SM. Corticosteroid use in COVID-19 patients: a systematic review and meta-analysis on clinical outcomes. Crit Care. 2020;24(s):696. https://doi.org/10.1186/s13054-020-03400-9.",
"volume": "24",
"year": "2020"
},
{
"DOI": "10.1186/s12943-021-01363-1",
"author": "Z Zong",
"doi-asserted-by": "publisher",
"first-page": "76",
"issue": "1",
"journal-title": "Mol Cancer.",
"key": "2850_CR33",
"unstructured": "Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer. 2021;20(1):76. https://doi.org/10.1186/s12943-021-01363-1.",
"volume": "20",
"year": "2021"
},
{
"DOI": "10.1056/NEJMoa2030340",
"author": "C Salama",
"doi-asserted-by": "publisher",
"first-page": "20",
"issue": "1",
"journal-title": "N Engl J Med.",
"key": "2850_CR34",
"unstructured": "Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2020;384(1):20–30. https://doi.org/10.1056/NEJMoa2030340.",
"volume": "384",
"year": "2020"
},
{
"DOI": "10.1002/jmv.26509",
"author": "M Aziz",
"doi-asserted-by": "publisher",
"first-page": "1620",
"issue": "3",
"journal-title": "J Med Virol.",
"key": "2850_CR35",
"unstructured": "Aziz M, Haghbin H, Abu Sitta E, et al. Efficacy of tocilizumab in COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(3):1620–30. https://doi.org/10.1002/jmv.26509.",
"volume": "93",
"year": "2021"
},
{
"DOI": "10.1038/s41418-021-00817-9",
"author": "A-G Goubet",
"doi-asserted-by": "publisher",
"first-page": "3297",
"issue": "12",
"journal-title": "Cell Death Different.",
"key": "2850_CR36",
"unstructured": "Goubet A-G, Dubuisson A, Geraud A, et al. Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis. Cell Death Different. 2021;28(12):3297–315. https://doi.org/10.1038/s41418-021-00817-9.",
"volume": "28",
"year": "2021"
},
{
"DOI": "10.1001/archinte.1982.00340160107022",
"author": "MJ Hauser",
"doi-asserted-by": "publisher",
"first-page": "527",
"issue": "3",
"journal-title": "Arch Intern Med.",
"key": "2850_CR37",
"unstructured": "Hauser MJ, Tabak J, Baier H. Survival of patients with Cancer in a medical critical care unit. Arch Intern Med. 1982;142(3):527–9. https://doi.org/10.1001/archinte.1982.00340160107022.",
"volume": "142",
"year": "1982"
},
{
"DOI": "10.1136/bmjnph-2021-000269",
"author": "C Espejo-Paeres",
"doi-asserted-by": "publisher",
"first-page": "285",
"issue": "1",
"journal-title": "BMJ Nutr Prev Health.",
"key": "2850_CR38",
"unstructured": "Espejo-Paeres C, Núñez-Gil IJ, Estrada V, et al. Impact of smoking on COVID-19 outcomes: a HOPE registry subanalysis. BMJ Nutr Prev Health. 2021;4(1):285–92. https://doi.org/10.1136/bmjnph-2021-000269.",
"volume": "4",
"year": "2021"
},
{
"DOI": "10.1002/jmv.25889",
"author": "Q Zhao",
"doi-asserted-by": "publisher",
"first-page": "1915",
"issue": "10",
"journal-title": "J Med Virol.",
"key": "2850_CR39",
"unstructured": "Zhao Q, Meng M, Kumar R, et al. The impact of COPD and smoking history on the severity of COVID-19: a systemic review and meta-analysis. J Med Virol. 2020;92(10):1915–21. https://doi.org/10.1002/jmv.25889.",
"volume": "92",
"year": "2020"
},
{
"DOI": "10.1093/ntr/ntaa082",
"author": "R Patanavanich",
"doi-asserted-by": "publisher",
"first-page": "1653",
"issue": "9",
"journal-title": "Nicotine Tob Res.",
"key": "2850_CR40",
"unstructured": "Patanavanich R, Glantz SA. Smoking Is Associated With COVID-19 Progression: A Meta-analysis. Nicotine Tob Res. 2020;22(9):1653–6. https://doi.org/10.1093/ntr/ntaa082.",
"volume": "22",
"year": "2020"
},
{
"DOI": "10.1016/j.devcel.2020.05.012",
"author": "JC Smith",
"doi-asserted-by": "publisher",
"first-page": "514",
"issue": "5",
"journal-title": "Dev Cell.",
"key": "2850_CR41",
"unstructured": "Smith JC, Sausville EL, Girish V, et al. Cigarette Smoke Exposure and Inflammatory Signaling Increase the Expression of the SARS-CoV-2 Receptor ACE2 in the Respiratory Tract. Dev Cell. 2020;53(5):514–529.e3. https://doi.org/10.1016/j.devcel.2020.05.012.",
"volume": "53",
"year": "2020"
},
{
"DOI": "10.1016/j.ejim.2020.03.014",
"author": "G Lippi",
"doi-asserted-by": "publisher",
"first-page": "107",
"journal-title": "Eur J Intern Med.",
"key": "2850_CR42",
"unstructured": "Lippi G, Henry BM. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19). Eur J Intern Med. 2020;75:107–8. https://doi.org/10.1016/j.ejim.2020.03.014.",
"volume": "75",
"year": "2020"
},
{
"DOI": "10.1016/j.ajem.2020.11.025",
"author": "L Szarpak",
"doi-asserted-by": "publisher",
"first-page": "638",
"journal-title": "Am J Emerg Med.",
"key": "2850_CR43",
"unstructured": "Szarpak L, Ruetzler K, Safiejko K, et al. Lactate dehydrogenase level as a COVID-19 severity marker. Am J Emerg Med. 2021;45:638–9. https://doi.org/10.1016/j.ajem.2020.11.025.",
"volume": "45",
"year": "2021"
},
{
"DOI": "10.1097/01.Ccm.0000256846.27192.7a",
"author": "L Lecuyer",
"doi-asserted-by": "publisher",
"first-page": "808",
"issue": "3",
"journal-title": "Crit Care Med.",
"key": "2850_CR44",
"unstructured": "Lecuyer L, Chevret S, Thiery G, Darmon M, Schlemmer B, Azoulay E. The ICU trial: a new admission policy for cancer patients requiring mechanical ventilation. Crit Care Med. 2007;35(3):808–14. https://doi.org/10.1097/01.Ccm.0000256846.27192.7a.",
"volume": "35",
"year": "2007"
},
{
"DOI": "10.1016/j.accpm.2020.08.001",
"author": "F Michard",
"doi-asserted-by": "publisher",
"first-page": "563",
"issue": "5",
"journal-title": "Anaesth Crit Care Pain Med.",
"key": "2850_CR45",
"unstructured": "Michard F, Malbrain ML, Martin GS, et al. Haemodynamic monitoring and management in COVID-19 intensive care patients: an international survey. Anaesth Crit Care Pain Med. 2020;39(5):563–9. https://doi.org/10.1016/j.accpm.2020.08.001.",
"volume": "39",
"year": "2020"
}
],
"reference-count": 45,
"references-count": 45,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcpulmmed.biomedcentral.com/articles/10.1186/s12890-024-02850-z"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pulmonary and Respiratory Medicine"
],
"subtitle": [],
"title": "Clinical characteristics and outcomes among critically ill patients with cancer and COVID-19-related acute respiratory failure",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "24"
}