Clinical efficacy analysis of paxlovid in children with hematological diseases infected with the omicron SARS-CoV-2 new variant
et al., Frontiers in Pediatrics, doi:10.3389/fped.2023.1160929, Apr 2023
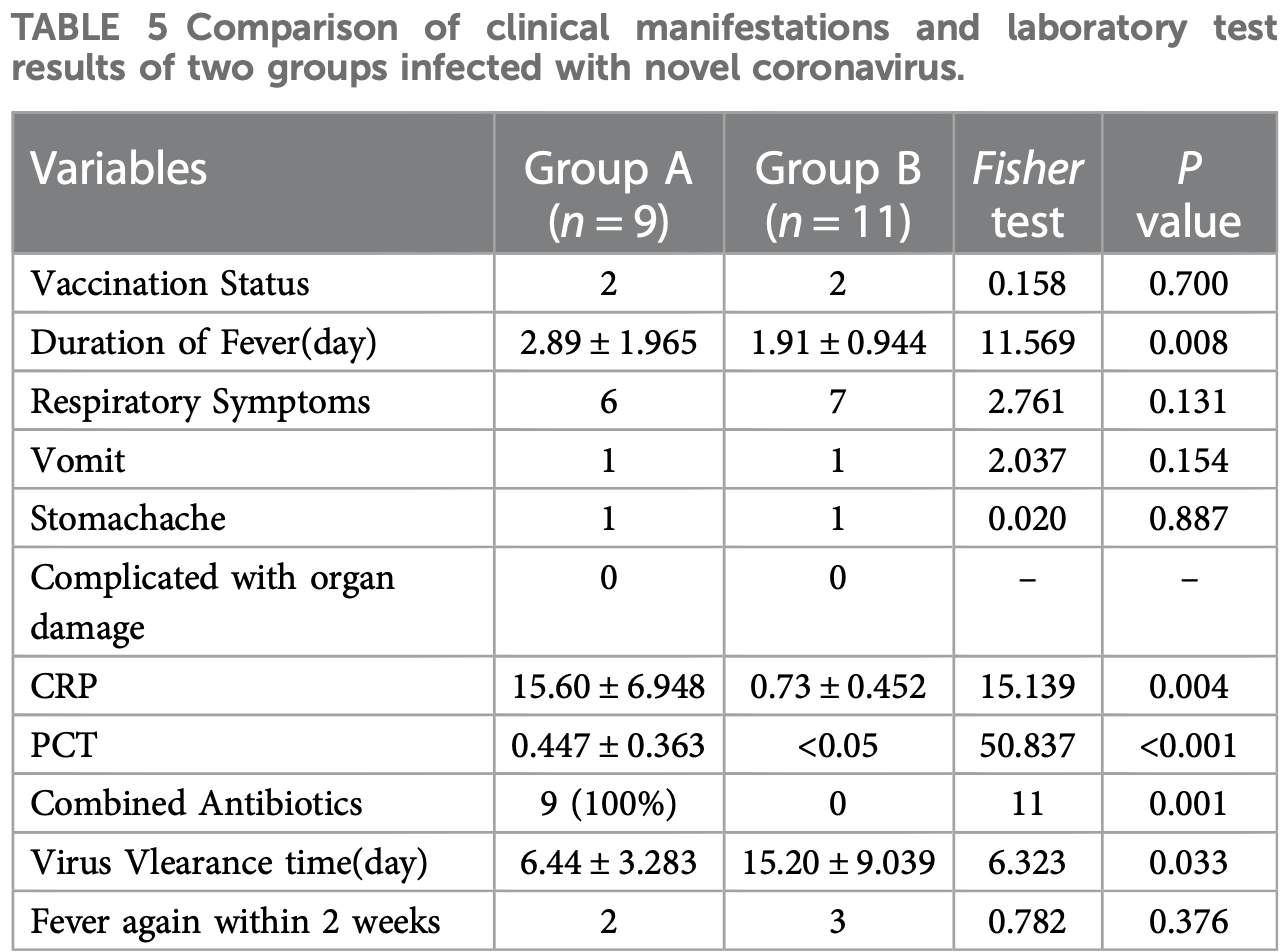
Retrospective 20 pediatric hematological disease patients in China, showing faster viral clearance with paxlovid, but slower resolution of fever.
Resistance. Variants may be resistant to paxlovid1-8. Use may promote the emergence of variants that weaken host immunity and potentially contribute to long COVID9. Confounding by contraindication. Hoertel et al. find that over 50% of patients that died had a contraindication for the use of Paxlovid10. Retrospective studies that do not exclude contraindicated patients may significantly overestimate efficacy. Black box warning. The FDA notes that severe, life-threatening, and/or fatal adverse reactions due to drug interactions have been reported in patients treated with paxlovid11. Kidney and liver injury. Studies show significantly increased risk of acute kidney injury12 and liver injury13,14. Viral rebound. Studies show significantly increased risk of replication-competent viral rebound15-17.
Standard of Care (SOC) for COVID-19 in the study country,
China, is average with moderate efficacy for approved treatments18.
|
resolution of fever, 51.3% higher, relative time 1.51, p = 0.008, treatment 9, control 11.
|
|
time to viral-, 57.6% lower, relative time 0.42, p = 0.03, treatment 9, control 11.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
1.
Zhou et al., Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system, Science Advances, doi:10.1126/sciadv.add7197.
2.
Moghadasi et al., Rapid resistance profiling of SARS-CoV-2 protease inhibitors, npj Antimicrobials and Resistance, doi:10.1038/s44259-023-00009-0.
3.
Jochmans et al., The Substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro Are Selected by a Protease Inhibitor In Vitro and Confer Resistance To Nirmatrelvir, mBio, doi:10.1128/mbio.02815-22.
4.
Lopez et al., SARS-CoV-2 Resistance to Small Molecule Inhibitors, Current Clinical Microbiology Reports, doi:10.1007/s40588-024-00229-6.
5.
Zvornicanin et al., Molecular Mechanisms of Drug Resistance and Compensation in SARS-CoV-2 Main Protease: The Interplay Between E166 and L50, bioRxiv, doi:10.1101/2025.01.24.634813.
6.
Vukovikj et al., Impact of SARS-CoV-2 variant mutations on susceptibility to monoclonal antibodies and antiviral drugs: a non-systematic review, April 2022 to October 2024, Eurosurveillance, doi:10.2807/1560-7917.ES.2025.30.10.2400252.
7.
Deschenes et al., Functional and structural characterization of treatment-emergent nirmatrelvir resistance mutations at low frequencies in the main protease (Mpro) reveals a unique evolutionary route for SARS-CoV-2 to gain resistance, The Journal of Infectious Diseases, doi:10.1093/infdis/jiaf294.
8.
Zhou (B) et al., SARS-CoV-2 Mpro inhibitor ensitrelvir: asymmetrical cross-resistance with nirmatrelvir and emerging resistance hotspots, Emerging Microbes & Infections, doi:10.1080/22221751.2025.2552716.
9.
Thomas et al., Nirmatrelvir-Resistant Mutations in SARS-CoV-2 Mpro Enhance Host Immune Evasion via Cleavage of NF-κB Essential Modulator, bioRxiv, doi:10.1101/2024.10.18.619137.
10.
Hoertel et al., Prevalence of Contraindications to Nirmatrelvir-Ritonavir Among Hospitalized Patients With COVID-19 at Risk for Progression to Severe Disease, JAMA Network Open, doi:10.1001/jamanetworkopen.2022.42140.
11.
FDA, Fact sheet for healthcare providers: emergency use authorization for paxlovid, www.fda.gov/media/155050/download.
12.
Kamo et al., Association of Antiviral Drugs for the Treatment of COVID-19 With Acute Renal Failure, In Vivo, doi:10.21873/invivo.13637.
13.
Wang et al., Development and validation of a nomogram to assess the occurrence of liver dysfunction in patients with COVID-19 pneumonia in the ICU, BMC Infectious Diseases, doi:10.1186/s12879-025-10684-1.
14.
Siby et al., Temporal Trends in Serious Adverse Events Associated with Oral Antivirals During the COVID-19 Pandemic: Insights from the FAERS Database (2020–2023), Open Forum Infectious Diseases, doi:10.1093/ofid/ofaf695.1825.
15.
Edelstein et al., SARS-CoV-2 virologic rebound with nirmatrelvir-ritonavir therapy, medRxiv, doi:10.1101/2023.06.23.23288598.
16.
Shah et al., SARS-CoV-2 infectious shedding and rebound among adults with and without oral antiviral use: two case-ascertained prospective household studies, The Lancet Microbe, doi:10.1016/j.lanmic.2025.101227.
Li et al., 25 Apr 2023, retrospective, China, peer-reviewed, 9 authors, study period 10 December, 2022 - 20 January, 2023.
Contact: chenchun@mail.sysu.edu.cn, xuehongman@sysush.com, chengyucai@sysush.com.
Clinical efficacy analysis of paxlovid in children with hematological diseases infected with the omicron SARS-CoV-2 new variant
Frontiers in Pediatrics, doi:10.3389/fped.2023.1160929
Objective: To summarize the clinical characteristics of children with hematological malignancies co-infected with novel coronavirus and explore the safety and effectiveness of Paxlovid treatment. Methods: From December 10, 2022, to January 20, 2023, the clinical data of children with hematological diseases diagnosed with novel coronavirus infection in the outpatient and emergency department of the Seventh Affiliated Hospital of Sun Yat-sen University were retrospectively analyzed. Results: According to whether to give paxlovid or not, it is divided into group A (paxlovid group) and group B (non-paxlovid group). The length of fever was 1-6 days in group A and 0-3 days in group B. The viral clearance time was shorter in group A than in group B. The inflammatory indexes CRP and PCT were significantly higher in group A than in group B (P < 0.05). Twenty patients were followed up for 1 month after leaving the hospital, and there were 5 cases of reappearance of fever, 1 case of increased sleep, 1 case of physical fatigue and 1 case of loss of appetite within 2 weeks. Conclusions: Paxlovid has no apparent adverse reactions in children 12 years old and younger with underlying hematological diseases infected with the new coronavirus. Focusing on the interaction between paxlovid and other drugs is necessary during the treatment.
Ethics statement The studies involving human participants were reviewed and approved by Ethics Committee of the Seventh Affiliated Hospital of Sun Yat-sen University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions CC, HX and YC designed research; YL and YL analyzed data, and wrote the manuscript; LW, HC and WW acquired data, collected clinical data. MT reviewed the data. CC interpreted the data and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Badri, Dutta, Coakley, Cohen, Ding et al., Pharmacokinetics and dose recommendations for cyclosporine and tacrolimus when coadministered with ABT-450, ombitasvir, and dasabuvir, Am J Transplant, doi:10.1111/ajt.13111
Bhardwaj, Joshi, Gaur, IoT-Based smart health monitoring system for COVID-19, SN Comput Sci, doi:10.1007/s42979-022-01015-1
Biomed, None, doi:10.1016/j.bj.2020.11.005
Cazalilla, COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of spanish group of transplant (GETMON/GETH), Pediatr Blood Cancer, doi:10.1002/pbc.28514
Cesaro, Ljungman, Mikulska, Hirsch, Lilienfeld-Toal et al., Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, doi:10.1038/s41375-022-01578-1
Creech, Anderson, Berthaud, Yildirim, Atz et al., Evaluation of mRNA-1273 COVID-19 vaccine in children 6 to 11 years of age, N Engl J Med, doi:10.1056/NEJMoa2203315
Diesch-Furlanetto, Gabriel, Zajac-Spychala, Cattoni, Hoeben et al., Late effects after haematopoietic stem cell transplantation in ALL, longterm follow-up and transition: a step into adult life, Front Pediatr, doi:10.3389/fped.2021.773895
Elens, Langman, Hesselink, Bergan, Moes et al., Pharmacologic treatment of transplant recipients infected with SARS-CoV-2: considerations regarding therapeutic drug monitoring and drug-drug interactions, Ther Drug Monit, doi:10.1097/FTD.0000000000000761
Fernandes, Inchakalody, Merhi, Mestiri, Taib et al., Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines, Ann Med, doi:10.1080/07853890.2022.2031274
Groll, Townsend, Desai, Azie, Jones et al., Drugdrug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4, Transpl Infect Dis, doi:10.1111/tid.12751
Haeusler, Ammann, Carlesse, Groll, Averbuch et al., SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: an analysis of 131 patients, Eur J Cancer, doi:10.1016/j.ejca.2021.09.027
Hammond, Leister-Tebbe, Gardner, Abreu, Wisemandle, Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19, N Engl J Med, doi:10.1056/NEJMoa2118542
Huang, Yin, Qin, Yu, Jiang et al., Case report: application of nirmatrelvir/ritonavir to treat COVID-19 in a severe aplastic anemia child after allogeneic hematopoietic stem cell transplantation, Front Pediatr, doi:10.3389/fped.2022.935118
Jayaweera, Perera, Gunawardana, Manatunge, Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy, Environ Res, doi:10.1016/j.envres.2020.109819
Kim, Chinese Guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever, Zhonghua Xue Ye Xue Za Zhi, doi:10.3760/cma.j.issn.0253-2727.2020.12.001
Koinuma, Nunomiya, Wada, Koyama, Suzuki, Concurrent treatment with a tumor necrosis factor-alpha inhibitor and veno-venous extracorporeal membrane oxygenation in a post-hematopoietic stem cell transplant patient with idiopathic pneumonia syndrome: a case report, J Intensive Care, doi:10.1186/s40560-014-0048-1
Levy, Bacharier, Bateman, Boulet, Brightling et al., Guidelines for the prevention and management of children and adolescents with COVID-19, Eur J Pediatr, doi:10.1007/s00431-022-04615-4
Li, None
Liu, Chen, Hu, Huang, Geng et al., An ultra-sensitive and specific nanoplasmonic-enhanced isothermal amplification platform for the ultrafast point-of-care testing of SARS-CoV-2, Chem Eng J, doi:10.1016/j.cej.2022.138822
Lucchini, Furness, Lawson, Gibson, Wynn et al., COVID-19 infection in paediatric recipients of allogeneic stem cell transplantation: the UK experience, Br J Haematol, doi:10.1111/bjh.17547
Magro, Zanella, Pescarolo, Castelli, Quiros-Roldan, Lopinavir/ ritonavir: repurposing an old drug for HIV infection in COVID-19 treatment
Malden, Hong, Lewin, Ackerson, Lipsitch et al., Hospitalization and emergency department encounters for COVID-19 after Li et
Najjar-Debbiny, Gronich, Weber, Khoury, Amar et al., Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients, Clin Infect Dis, doi:10.1093/cid/ciac443
Rana, Kant, Huirem, Bohra, Ganguly, Omicron variant: current insights and future directions, Microbiol Res, doi:10.1016/j.micres.2022.127204
Robert, Wong, Wright, Lemaitre, Budde et al., Therapeutic drug monitoring and dosage adjustments of immunosuppressive drugs when combined with nirmatrelvir/ritonavir in patients with COVID-19, Cochrane Database Syst Rev, doi:10.1097/FTD.0000000000001014
Sanft, Day, Peterson, Rodriguez, Ansbaugh et al., NCCN Guidelines ® insights: survivorship, version 1.2022, J Natl Compr Canc Netw, doi:10.6004/jnccn.2022.0052
Saravolatz, Depcinski, Sharma, Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs, Clin Infect Dis, doi:10.1093/cid/ciac180
Schwartz, Does ivermectin have a place in the treatment of mild COVID-19?, New Microbes New Infect, doi:10.1016/j.nmni.2022.100985
Sette, Crotty, Adaptive immunity to SARS-CoV-2 and COVID-19, Cell, doi:10.1016/j.cell.2021.01.007
Shah, Joyce, Plumb, Sahakian, Feldstein et al., Paxlovid associated with decreased hospitalization rate among adults with COVID-19-united States, MMWR Morb Mortal Wkly Rep, doi:10.15585/mmwr.mm7148e2
Siddiqi, Mehra, COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal, J Heart Lung Transplant, doi:10.1016/j.healun.2020.03.012
Tiseo, Barbieri, Galfo, Occhineri, Matucci et al., Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a realworld cohort of outpatients with COVID-19 at high risk of progression: the PISA outpatient clinic experience, Infect Dis Ther, doi:10.1007/s40121-022-00729-2
Tulimilli, Dallavalasa, Basavaraju, Rao, Chikkahonnaiah et al., Variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and vaccine effectiveness. Vaccines (Basel), doi:10.3390/vaccines10101751
Vicent, Martinez, Del Castillo, Molina, Sisini, None
Vogel, Voigt, Michaelis, Sudhop, Wolff et al., Management of drug-to-drug interactions between cyclosporine A and the proteaseinhibitor lopinavir/ritonavir in liver-transplanted HIV-infected patients, Liver Transpl, doi:10.1002/lt.20165
Yan, Zhou, Zhu, Chen, Lu et al., The feasibility, safety, and efficacy of paxlovid treatment in SARS-CoV-2-infected children aged 6-14 years: a cohort study, Ann Transl Med. (2022), doi:10.21037/atm-22-2791
Zimmermann, Curtis, Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections, Arch Dis Child, doi:10.1136/archdischild-2020-320338
DOI record:
{
"DOI": "10.3389/fped.2023.1160929",
"ISSN": [
"2296-2360"
],
"URL": "http://dx.doi.org/10.3389/fped.2023.1160929",
"abstract": "<jats:sec><jats:title>Objective</jats:title><jats:p>To summarize the clinical characteristics of children with hematological malignancies co-infected with novel coronavirus and explore the safety and effectiveness of Paxlovid treatment.</jats:p></jats:sec><jats:sec><jats:title>Methods</jats:title><jats:p>From December 10, 2022, to January 20, 2023, the clinical data of children with hematological diseases diagnosed with novel coronavirus infection in the outpatient and emergency department of the Seventh Affiliated Hospital of Sun Yat-sen University were retrospectively analyzed.</jats:p></jats:sec><jats:sec><jats:title>Results</jats:title><jats:p>According to whether to give paxlovid or not, it is divided into group A (paxlovid group) and group B (non-paxlovid group). The length of fever was 1–6 days in group A and 0–3 days in group B. The viral clearance time was shorter in group A than in group B. The inflammatory indexes CRP and PCT were significantly higher in group A than in group B (<jats:italic>P </jats:italic>&lt; 0.05). Twenty patients were followed up for 1 month after leaving the hospital, and there were 5 cases of reappearance of fever, 1 case of increased sleep, 1 case of physical fatigue and 1 case of loss of appetite within 2 weeks.</jats:p></jats:sec><jats:sec><jats:title>Conclusions</jats:title><jats:p>Paxlovid has no apparent adverse reactions in children 12 years old and younger with underlying hematological diseases infected with the new coronavirus. Focusing on the interaction between paxlovid and other drugs is necessary during the treatment.</jats:p></jats:sec>",
"alternative-id": [
"10.3389/fped.2023.1160929"
],
"author": [
{
"affiliation": [],
"family": "Li",
"given": "Yixian",
"sequence": "first"
},
{
"affiliation": [],
"family": "Liu",
"given": "Yong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wen",
"given": "Luping",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Hui",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Wang",
"given": "Wenqing",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Tian",
"given": "Mengyao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cheng",
"given": "Yucai",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Xue",
"given": "Hongman",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Chen",
"given": "Chun",
"sequence": "additional"
}
],
"container-title": "Frontiers in Pediatrics",
"container-title-short": "Front. Pediatr.",
"content-domain": {
"crossmark-restriction": true,
"domain": [
"frontiersin.org"
]
},
"created": {
"date-parts": [
[
2023,
4,
25
]
],
"date-time": "2023-04-25T05:17:40Z",
"timestamp": 1682399860000
},
"deposited": {
"date-parts": [
[
2023,
4,
25
]
],
"date-time": "2023-04-25T05:17:45Z",
"timestamp": 1682399865000
},
"indexed": {
"date-parts": [
[
2023,
4,
26
]
],
"date-time": "2023-04-26T05:09:39Z",
"timestamp": 1682485779979
},
"is-referenced-by-count": 0,
"issued": {
"date-parts": [
[
2023,
4,
25
]
]
},
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0/",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2023,
4,
25
]
],
"date-time": "2023-04-25T00:00:00Z",
"timestamp": 1682380800000
}
}
],
"link": [
{
"URL": "https://www.frontiersin.org/articles/10.3389/fped.2023.1160929/full",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "1965",
"original-title": [],
"prefix": "10.3389",
"published": {
"date-parts": [
[
2023,
4,
25
]
]
},
"published-online": {
"date-parts": [
[
2023,
4,
25
]
]
},
"publisher": "Frontiers Media SA",
"reference": [
{
"DOI": "10.3390/vaccines10101751",
"article-title": "Variants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and vaccine effectiveness",
"author": "Tulimilli",
"doi-asserted-by": "publisher",
"journal-title": "Vaccines (Basel)",
"key": "B1",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1016/j.micres.2022.127204",
"article-title": "Omicron variant: current insights and future directions",
"author": "Rana",
"doi-asserted-by": "publisher",
"first-page": "127204",
"journal-title": "Microbiol Res",
"key": "B2",
"volume": "265",
"year": "2022"
},
{
"DOI": "10.1016/j.healun.2020.03.012",
"article-title": "COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal",
"author": "Siddiqi",
"doi-asserted-by": "publisher",
"first-page": "405",
"journal-title": "J Heart Lung Transplant",
"key": "B3",
"volume": "39",
"year": "2020"
},
{
"DOI": "10.1016/j.envres.2020.109819",
"article-title": "Transmission of COVID-19 virus by droplets and aerosols: a critical review on the unresolved dichotomy",
"author": "Jayaweera",
"doi-asserted-by": "publisher",
"first-page": "109819",
"journal-title": "Environ Res",
"key": "B4",
"volume": "188",
"year": "2020"
},
{
"DOI": "10.1007/s42979-022-01015-1",
"article-title": "IoT-Based smart health monitoring system for COVID-19",
"author": "Bhardwaj",
"doi-asserted-by": "publisher",
"first-page": "137",
"journal-title": "SN Comput Sci",
"key": "B5",
"volume": "3",
"year": "2022"
},
{
"DOI": "10.1080/07853890.2022.2031274",
"article-title": "Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines",
"author": "Fernandes",
"doi-asserted-by": "publisher",
"first-page": "524",
"journal-title": "Ann Med",
"key": "B6",
"volume": "54",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac443",
"article-title": "Effectiveness of paxlovid in reducing severe COVID-19 and mortality in high risk patients",
"author": "Najjar-Debbiny",
"doi-asserted-by": "publisher",
"journal-title": "Clin Infect Dis",
"key": "B7",
"volume": "76",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2118542",
"article-title": "Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19",
"author": "Hammond",
"doi-asserted-by": "publisher",
"first-page": "1397",
"journal-title": "N Engl J Med",
"key": "B8",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1093/cid/ciac180",
"article-title": "Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs",
"author": "Saravolatz",
"doi-asserted-by": "publisher",
"first-page": "165",
"journal-title": "Clin Infect Dis",
"key": "B9",
"volume": "76",
"year": "2023"
},
{
"DOI": "10.15585/mmwr.mm7125e2",
"article-title": "Hospitalization and emergency department encounters for COVID-19 after paxlovid treatment—california, December 2021-may 2022",
"author": "Malden",
"doi-asserted-by": "publisher",
"first-page": "830",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "B10",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.21037/atm-22-2791",
"article-title": "The feasibility, safety, and efficacy of paxlovid treatment in SARS-CoV-2-infected children aged 6-14 years: a cohort study",
"author": "Yan",
"doi-asserted-by": "publisher",
"first-page": "619",
"journal-title": "Ann Transl Med",
"key": "B11",
"volume": "10",
"year": "2022"
},
{
"key": "B12",
"year": "2019"
},
{
"DOI": "10.1016/j.cej.2022.138822",
"article-title": "An ultra-sensitive and specific nanoplasmonic-enhanced isothermal amplification platform for the ultrafast point-of-care testing of SARS-CoV-2",
"author": "Liu",
"doi-asserted-by": "publisher",
"first-page": "138822",
"journal-title": "Chem Eng J",
"key": "B13",
"volume": "451",
"year": "2023"
},
{
"DOI": "10.6004/jnccn.2022.0052",
"article-title": "NCCN Guidelines® insights: survivorship, version 1.2022",
"author": "Sanft",
"doi-asserted-by": "publisher",
"first-page": "1080",
"journal-title": "J Natl Compr Canc Netw",
"key": "B14",
"volume": "20",
"year": "2022"
},
{
"DOI": "10.5395/rde.2017.42.2.152",
"article-title": "Statistical notes for clinical researchers: chi-squared test and Fisher's Exact test",
"author": "Kim",
"doi-asserted-by": "publisher",
"first-page": "152",
"journal-title": "Restor Dent Endod",
"key": "B15",
"volume": "42",
"year": "2017"
},
{
"DOI": "10.3760/cma.j.issn.0253-2727.2020.12.001",
"article-title": "Chinese Guidelines for the clinical application of antibacterial drugs for agranulocytosis with fever (2020)",
"doi-asserted-by": "publisher",
"first-page": "969",
"journal-title": "Zhonghua Xue Ye Xue Za Zhi",
"key": "B16",
"volume": "41",
"year": "2020"
},
{
"DOI": "10.1136/archdischild-2020-320338",
"article-title": "Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections",
"author": "Zimmermann",
"doi-asserted-by": "publisher",
"journal-title": "Arch Dis Child",
"key": "B17",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2021.01.007",
"article-title": "Adaptive immunity to SARS-CoV-2 and COVID-19",
"author": "Sette",
"doi-asserted-by": "publisher",
"first-page": "861",
"journal-title": "Cell",
"key": "B18",
"volume": "184",
"year": "2021"
},
{
"DOI": "10.3389/fped.2021.773895",
"article-title": "Late effects after haematopoietic stem cell transplantation in ALL, long-term follow-up and transition: a step into adult life",
"author": "Diesch-Furlanetto",
"doi-asserted-by": "publisher",
"first-page": "773895",
"journal-title": "Front Pediatr",
"key": "B19",
"volume": "9",
"year": "2021"
},
{
"DOI": "10.1186/s40560-014-0048-1",
"article-title": "Concurrent treatment with a tumor necrosis factor-alpha inhibitor and veno-venous extracorporeal membrane oxygenation in a post-hematopoietic stem cell transplant patient with idiopathic pneumonia syndrome: a case report",
"author": "Koinuma",
"doi-asserted-by": "publisher",
"first-page": "48",
"journal-title": "J Intensive Care",
"key": "B20",
"volume": "2",
"year": "2014"
},
{
"DOI": "10.1016/j.ejca.2021.09.027",
"article-title": "SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: an analysis of 131 patients",
"author": "Haeusler",
"doi-asserted-by": "publisher",
"first-page": "78",
"journal-title": "Eur J Cancer",
"key": "B21",
"volume": "159",
"year": "2021"
},
{
"DOI": "10.1007/s00431-022-04615-4",
"article-title": "Guidelines for the prevention and management of children and adolescents with COVID-19",
"author": "Levy",
"doi-asserted-by": "publisher",
"first-page": "4019",
"journal-title": "Eur J Pediatr",
"key": "B22",
"volume": "181",
"year": "2022"
},
{
"DOI": "10.1056/NEJMoa2203315",
"article-title": "Evaluation of mRNA-1273 COVID-19 vaccine in children 6 to 11 years of age",
"author": "Creech",
"doi-asserted-by": "publisher",
"first-page": "2011",
"journal-title": "N Engl J Med",
"key": "B23",
"volume": "386",
"year": "2022"
},
{
"DOI": "10.1007/s40121-022-00729-2",
"article-title": "Efficacy and safety of nirmatrelvir/ritonavir, molnupiravir, and remdesivir in a real-world cohort of outpatients with COVID-19 at high risk of progression: the PISA outpatient clinic experience",
"author": "Tiseo",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Infect Dis Ther",
"key": "B24",
"volume": "12",
"year": "2022"
},
{
"DOI": "10.15585/mmwr.mm7148e2",
"article-title": "Paxlovid associated with decreased hospitalization rate among adults with COVID-19—united States, April-September 2022",
"author": "Shah",
"doi-asserted-by": "publisher",
"first-page": "1531",
"journal-title": "MMWR Morb Mortal Wkly Rep",
"key": "B25",
"volume": "71",
"year": "2022"
},
{
"DOI": "10.1038/s41375-022-01578-1",
"article-title": "Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European conference on infections in leukaemia (ECIL 9)",
"author": "Cesaro",
"doi-asserted-by": "publisher",
"first-page": "1467",
"journal-title": "Leukemia",
"key": "B26",
"volume": "36",
"year": "2022"
},
{
"DOI": "10.1111/bjh.17547",
"article-title": "COVID-19 infection in paediatric recipients of allogeneic stem cell transplantation: the UK experience",
"author": "Lucchini",
"doi-asserted-by": "publisher",
"first-page": "e74",
"journal-title": "Br J Haematol",
"key": "B27",
"volume": "194",
"year": "2021"
},
{
"DOI": "10.1002/pbc.28514",
"article-title": "COVID-19 in pediatric hematopoietic stem cell transplantation: the experience of spanish group of transplant (GETMON/GETH)",
"author": "Vicent",
"doi-asserted-by": "publisher",
"first-page": "e28514",
"journal-title": "Pediatr Blood Cancer",
"key": "B28",
"volume": "67",
"year": "2020"
},
{
"DOI": "10.1016/j.nmni.2022.100985",
"article-title": "Does ivermectin have a place in the treatment of mild COVID-19?",
"author": "Schwartz",
"doi-asserted-by": "publisher",
"first-page": "100985",
"journal-title": "New Microbes New Infect",
"key": "B29",
"volume": "46",
"year": "2022"
},
{
"DOI": "10.3389/fped.2022.935118",
"article-title": "Case report: application of nirmatrelvir/ritonavir to treat COVID-19 in a severe aplastic anemia child after allogeneic hematopoietic stem cell transplantation",
"author": "Huang",
"doi-asserted-by": "publisher",
"first-page": "935118",
"journal-title": "Front Pediatr",
"key": "B30",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1097/FTD.0000000000000761",
"article-title": "Pharmacologic treatment of transplant recipients infected with SARS-CoV-2: considerations regarding therapeutic drug monitoring and drug-drug interactions",
"author": "Elens",
"doi-asserted-by": "publisher",
"first-page": "360",
"journal-title": "Ther Drug Monit",
"key": "B31",
"volume": "42",
"year": "2020"
},
{
"DOI": "10.1111/tid.12751",
"article-title": "Drug-drug interactions between triazole antifungal agents used to treat invasive aspergillosis and immunosuppressants metabolized by cytochrome P450 3A4",
"author": "Groll",
"doi-asserted-by": "publisher",
"journal-title": "Transpl Infect Dis",
"key": "B32",
"volume": "19",
"year": "2017"
},
{
"DOI": "10.1016/j.bj.2020.11.005",
"article-title": "Lopinavir/ritonavir: repurposing an old drug for HIV infection in COVID-19 treatment",
"author": "Magro",
"doi-asserted-by": "publisher",
"first-page": "43",
"journal-title": "Biomed J",
"key": "B33",
"volume": "44",
"year": "2021"
},
{
"DOI": "10.1111/ajt.13111",
"article-title": "Pharmacokinetics and dose recommendations for cyclosporine and tacrolimus when coadministered with ABT-450, ombitasvir, and dasabuvir",
"author": "Badri",
"doi-asserted-by": "publisher",
"first-page": "1313",
"journal-title": "Am J Transplant",
"key": "B34",
"volume": "15",
"year": "2015"
},
{
"DOI": "10.1002/14651858.CD007893.pub2",
"article-title": "Effect of cyclosporine on blood pressure",
"author": "Robert",
"doi-asserted-by": "publisher",
"first-page": "Cd007893",
"journal-title": "Cochrane Database Syst Rev",
"key": "B35",
"volume": "1",
"year": "2010"
},
{
"DOI": "10.1097/FTD.0000000000001014",
"article-title": "Therapeutic drug monitoring and dosage adjustments of immunosuppressive drugs when combined with nirmatrelvir/ritonavir in patients with COVID-19",
"author": "Lemaitre",
"doi-asserted-by": "publisher",
"first-page": "191",
"journal-title": "Ther Drug Monit",
"key": "B36",
"volume": "45",
"year": "2022"
},
{
"DOI": "10.1002/lt.20165",
"article-title": "Management of drug-to-drug interactions between cyclosporine A and the protease-inhibitor lopinavir/ritonavir in liver-transplanted HIV-infected patients",
"author": "Vogel",
"doi-asserted-by": "publisher",
"first-page": "939",
"journal-title": "Liver Transpl",
"key": "B37",
"volume": "10",
"year": "2004"
}
],
"reference-count": 37,
"references-count": 37,
"relation": {},
"resource": {
"primary": {
"URL": "https://www.frontiersin.org/articles/10.3389/fped.2023.1160929/full"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Pediatrics, Perinatology and Child Health"
],
"subtitle": [],
"title": "Clinical efficacy analysis of paxlovid in children with hematological diseases infected with the omicron SARS-CoV-2 new variant",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.3389/crossmark-policy",
"volume": "11"
}