The role of nutritional support with probiotics in outpatients with symptomatic acute respiratory tract infections: a multicenter, randomized, double-blind, placebo-controlled dietary study
et al., BMC Nutrition, doi:10.1186/s40795-023-00816-8, NCT04907877, Jan 2024
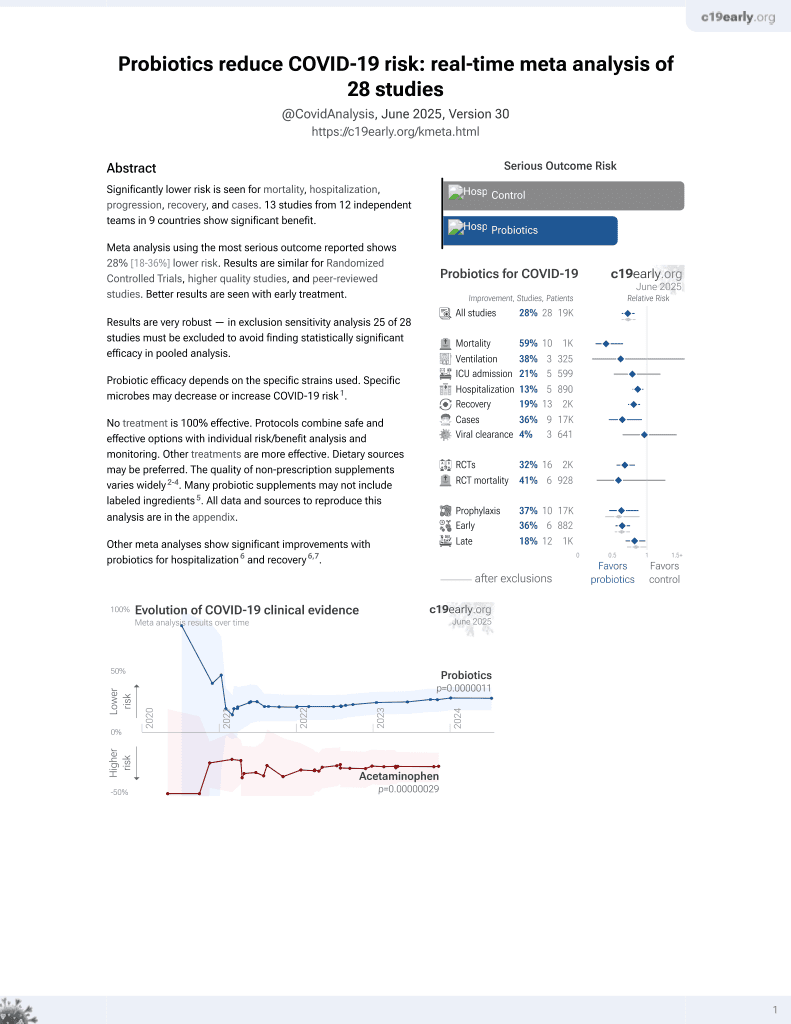
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
RCT 73 outpatients with mild COVID-19 showing improved recovery and increased RBD/spike antibody response with 28 days of a multi-strain probiotic (Bifidobacterium (B.) lactis BI040, B. longum BL020, Lactobacillus (L) rhamnosus LR110, L. casei LC130, L. acidophilus LA120, 5 billion CFU total).
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
|
WHO score >1, 60.3% lower, RR 0.40, p = 0.02, treatment 6 of 34 (17.6%), control 16 of 36 (44.4%), NNT 3.7.
|
|
recovery time, 21.4% lower, relative time 0.79, p = 0.04, treatment 34, control 36.
|
|
PCFS ≥1, 67.8% lower, RR 0.32, p = 0.008, treatment 5 of 34 (14.7%), control 16 of 35 (45.7%), NNT 3.2, long COVID, Supplementary Table 1.
|
| Effect extraction follows pre-specified rules prioritizing more serious outcomes. Submit updates |
Kolesnyk et al., 4 Jan 2024, Double Blind Randomized Controlled Trial, placebo-controlled, Ukraine, peer-reviewed, 10 authors, study period November 2021 - June 2022, trial NCT04907877 (history).
Contact: mediana.statistics@gmail.com.
The role of nutritional support with probiotics in outpatients with symptomatic acute respiratory tract infections: a multicenter, randomized, double-blind, placebo-controlled dietary study
BMC Nutrition, doi:10.1186/s40795-023-00816-8
Background A number of laboratory data and clinical studies have shown that probiotic bacteria may be beneficial in respiratory viral diseases. We investigated the role of probiotics in coronavirus disease-19 (COVID -19), post-disease symptoms, and humoral immune responses to viral antigens. Methods This was a randomized, double-blind, placebo-controlled, prospective, multicenter study. We included symptomatic patients aged 18-65 years without risk of severe disease, and positive antigen/PCR test for SARS-CoV-2. Patients received (Bifidobacterium (B.) lactis BI040, B. longum BL020, Lactobacillus (L) rhamnosus LR110, L. casei LC130, L. acidophilus LA120, 5 billion CFU total) or placebo 1 capsule a day for 28 days and recorded symptoms. Three months later patients completed Post-COVID-19 Questionnaire (PCQ-19). On days 0-5 and 28-35, blood was sampled for IgG to nucleocapsid protein (NCP) and receptor binding domain (RBD)/spike 1 (S1) protein. The primary outcome measure was a patient global symptom score on day 10 of observation. The difference between groups was assessed using the Mann-Whitney U test.
Results Seventy-three patients were assessed for clinical endpoints and 44 patients were evaluated for antibody production. At day 10, the median global symptom score (interquartile range) was lower in the probiotic group (0.0 (0.0-2.0) vs. 2.0 (1.0-5.0), P < 0.05). The probiotic group had a shorter duration of fatigue and anxiety after COVID -19 (P < 0.05) and a greater change in IgG concentration on RBD/S1 (225.9 vs. 105.6 binding antibody units/mL, P < 0.05). Conclusions Use of probiotics alleviates acute and post-disease symptoms, and improves humoral immune response to viral antigens.
Supplementary Information The online version contains supplementary material available at https:// doi. org/ 10. 1186/ s40795-023-00816-8. Additional file 1: Supplemental Table 1 . The Post-COVID-19 Questionnaire and Post-COVID-19 Functional Scale data after a 3-month follow-up.
Authors' contributions POK, IHP, LPS, ZРH, OSL, NRH were responsible for the patient enrollment, data collection and reviewing the report. NOI, ZLS, OIM were responsible for the data collection and reviewing the report. SVG was responsible for designing and writing the protocol, extracting and analyzing data, interpreting results, creating tables and figures, writing the report.
Funding The study was sponsored by Nordic Biotic Sp. z o.o. (Warsaw, Poland). The sponsor was not involved in the study hypothesis/design, implementation, analysis, and interpretation. The funding was transparent, acknowledged, and appropriately recognized throughout all stages of study. The study was conducted in a full academic independence to report and publish all the findings.
Availability of data and materials The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations Ethics approval and consent to participate Approvals were obtained from ethical committees at all study sites (Lviv Oblast Center for Disease Control and Prevention Ministry of Health of Ukraine, protocol #2 of 20 Apr 2021; Lviv Municipal Non-Profit Enterprise Third..
References
Bradley, Finsterbusch, Schnepf, Crotta, Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection, Cell Rep, doi:10.1016/j.celrep.2019.05.105
Campbell, Archer, Laurenson-Schafer, Jinnai, Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021, Euro Surveill, doi:10.2807/1560-7917.ES.2021.26.24.2100509
Carfì, Bernabei, Landi, Persistent symptoms in patients after acute COVID-19, JAMA, doi:10.1001/jama.2020.12603
Chai, Burwinkel, Wang, Antiviral effects of a probiotic Enterococcusfaecium strain against transmissible gastroenteritis coronavirus, Arch Virol, doi:10.1007/s00705-012-1543-0
Daliri, Oh, Lee, Psychobiotics; a promise for neurodevelopmental therapy, J Probiotics Health
De Boeck, Cauwenberghs, Spacova, Gehrmann, Randomized, double-blind, placebo-controlled trial of a throat spray with selected lactobacilli in COVID-19 outpatients, Microbiol Spectr, doi:10.1128/spectrum.01682-22
De Weerth, Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis, Neurosci Biobehav Rev
Dinç, Demirci, Özdemir, Sirekbasan, Anti-SARS-CoV-2 IgG and neutralizing antibody levels in patients with past COVID-19 infection: a longitudinal study, Balkan Med J, doi:10.4274/balkanmedj.galenos.2022.2021-8-131
Endam, Tremblay, Filali, Desrosiers, Intranasal application of Lactococcuslactis W 136 bacteria early in SARS-Cov-2 infection may have a beneficial immunomodulatory effect: a proof-of-concept study, medRxiv, doi:10.1101/2021.04.18.21255699
Ettorre, Ceccarelli, Marazzato, Campagna, Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19, Front Med, doi:10.3389/fmed.2020.00389
Fendrick, Saint, Brook, Jacobs, Diagnosis and treatment of upper respiratory tract infections in the primary care setting, Clin Ther, doi:10.1016/s0149-2918(01)80137-5
Flaherty, Immunogenicity and antigenicity
Fülling, Dinan, Cryan, Gut microbe to brain signaling: what happens in vagus, Neuron
Geva-Zatorsky, Sefik, Kua, Pasman, Mining the human gut microbiota for immunomodulatory organisms, Cell, doi:10.1016/j.cell.2017.01.022
Gutiérrez-Castrellón, Gandara-Martí, Abreu, Abreu, Nieto-Rufino, Probiotic improves symptomatic and viral clearance in Covid-19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial, Gut Microbes, doi:10.1080/19490976.2021.2018899
He, Wang, Li, Shi, Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections, Front Microbiol, doi:10.3389/fmicb.2020.01302
He, Wen, Yao, Xu, Gut-lung axis: the microbial contributions and clinical implications, Crit Rev Microbiol, doi:10.1080/1040841X.2016.1176988
Hori, Kiyoshima, Shida, Yasui, Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacilluscasei strain Shirota, Clin Diagn Lab Immunol, doi:10.1128/cdli.9.1.105-108.2002.31
Huang, Wang, Li, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China, Lancet, doi:10.1016/S0140-6736(20)30183-5
Ichinohe, Pang, Kumamoto, Peaper, Microbiota regulates immune defense against respiratory tract influenza A virus infection, Proc Natl Acad Sci, doi:10.1073/pnas.1019378108
Incze, Grady, Gupta, I have a cold-what do i need to know?, JAMA Intern Med, doi:10.1001/jamainternmed.2018.2621
Jin, Ren, Li, Gao, Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019, EClinicalMedicine, doi:10.1016/j.eclinm.2021.100986
Johnson, Xie, Bailey, Kalveram, Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis, Nature, doi:10.1038/s41586-021-03237-4
Klok, Boon, Barco, The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19, Eur Respir J, doi:10.1183/13993003.01494-2020
Lee, Mazmanian, Has the microbiota played a critical role in the evolution of the adaptive immune system?, Science, doi:10.1126/science.1195568
Liu, Walsh, Sheehan, Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials, Neurosci Biobehav Rev, doi:10.1016/j.neubiorev.2019.03.023
Liu, Wang, Nair, Yu, Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike, Nature, doi:10.1038/s41586-020-2571-7
Maeda, Nakamura, Hirose, Murosaki, Oral administration of heat-killed Lactobacillusplantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice, Int Immunopharmacol, doi:10.1016/j.intimp.2009.04.015
Maffei, Montemiglio, Vitagliano, Fedele, The nuts and bolts of SARS-CoV-2 spike receptor-binding domain heterologous expression, Biomolecules, doi:10.3390/biom11121812
Navarro-Lopez, Hernandez-Belmonte, Perez, Ayo-Gonzalez, Oral intake of Kluyveromycesmarxianus B0399 plus Lactobacillusrhamnosus CECT 30579 to mitigate symptoms in COVID-19 patients: a randomized open label clinical trial, Med Microecol, doi:10.1016/j.medmic.2022.100061
Nyberg, Ferguson, Nash, Webster, Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study, Lancet, doi:10.1016/S0140-6736(22)00462-7
Olaimat, Aolymat, Holy, Ayyash, The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19, NPJ Sci Food, doi:10.1038/s41538-020-00078-9
Pan, Mu, Yang, Sun, Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, crosssectional, multicenter study, Am J Gastroenterol, doi:10.14309/ajg.0000000000000620
Potenza, Siciliano, Petruzziello, COVID-19 pneumonia and gut inflammation: the role of a mix of three probiotic strains in reducing inflammatory markers and need for oxygen support, J Clin Med, doi:10.3390/jcm11133758
Premraj, Kannapadi, Briggs, Seal, Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis, J Neurol Sci, doi:10.1016/j.jns.2022.120162
Reino-Gelardo, Palop-Cervera, Aparisi-Valero, Espinosa-San, Miguel, Effect of an immune-boosting, antioxidant and anti-inflammatory food supplement in hospitalized COVID-19 patients: a prospective randomized pilot study, Nutrients, doi:10.3390/nu15071736
Sampson, Mazmanian, Control of brain development, function, and behavior by the microbiome, Cell Host Microbe
Shida, Sato, Iizuka, Hoshi, Daily intake of fermented milk with Lactobacilluscasei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers, Eur J Nutr, doi:10.1007/s00394-015-1056-1
Smyk, Janik, Portincasa, Milkiewicz, COVID-19: focus on the lungs but do not forget the gastrointestinal tract, Eur J Clin Invest, doi:10.1111/eci.13276
Tonetti, Clua, Fukuyama, Marcial, The ability of postimmunobiotics from L.rhamnosus CRL1505 to protect against respiratory syncytial virus and pneumococcal super-infection is a strain-dependent characteristic, Microorganisms, doi:10.3390/microorganisms10112185
Trompette, Gollwitzer, Pattaroni, Lopez-Mejia, Ic, Dietary fiber confers protection against flu by shaping Ly6c-patrolling monocyte hematopoiesis and CD8+ T cell metabolism, Immunity, doi:10.1016/j.immuni.2018.04.022
Vaezi, Ravanshad, Rad, Zarrinfar, Kabiri, The effect of synbiotic adjunct therapy on clinical and paraclinical outcomes in hospitalized COVID-19 patients: a randomized placebo-controlled trial, J Med Virol, doi:10.1002/jmv.28463
Van Tassell, Miller, Lactobacillus adhesion to mucus, Nutrients, doi:10.3390/nu3050613
Wang, Hu, Hu, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China JAMA, doi:10.1001/jama.2020.1585
Wang, Wang, Lu, Qiu, The efficacy of probiotics in patients with severe COVID-19, Ann Palliat Med, doi:10.21037/apm-21-3373
Westermann, Gleinser, Corr, Riedel, A critical evaluation of bifidobacterial adhesion to the host tissue, Front Microbiol, doi:10.3389/fmicb.2016.01220
Winkler, De Vrese, Ch, Schrezenmeir, Effect of a dietary supplement containing probiotic bacteria plus vitamins and minerals on common cold infections and cellular immune parameters, Int J Clin Pharmacol Ther, doi:10.5414/cpp43318
Yang, Yu, Xu, Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a singlecentered, retrospective, observational study, Lancet Respir Med, doi:10.1016/S2213-2600(20)30079-5
Yeoh, Zuo, Lui, Zhang, Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19, Gut, doi:10.1136/gutjnl-2020-323020
Zeng, Liu, Ma, Zhao, Biochemical characterization of SARS-CoV-2 nucleocapsid protein, Biochem Biophys Res Commun, doi:10.1016/j.bbrc.2020.04.136
Zhang, Han, Li, Chen, Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19, Therap Adv Gastroenterol, doi:10.1177/17562848211035670
Zhang, Yeh, Ding, Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate, Synth Syst Biotechnol, doi:10.1016/j.synbio.2018.03.001
Zhao, Dong, Hao, Probiotics for preventing acute upper respiratory tract infections, Cochrane Database Syst Rev, doi:10.1002/14651858.CD006895.pub4
Zhou, Yu, Du, Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study, doi:10.1016/S0140-6736(20)30566-3
DOI record:
{
"DOI": "10.1186/s40795-023-00816-8",
"ISSN": [
"2055-0928"
],
"URL": "http://dx.doi.org/10.1186/s40795-023-00816-8",
"abstract": "<jats:title>Abstract</jats:title><jats:sec>\n <jats:title>Background</jats:title>\n <jats:p>A number of laboratory data and clinical studies have shown that probiotic bacteria may be beneficial in respiratory viral diseases. We investigated the role of probiotics in coronavirus disease-19 (COVID -19), post-disease symptoms, and humoral immune responses to viral antigens.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Methods</jats:title>\n <jats:p>This was a randomized, double-blind, placebo-controlled, prospective, multicenter study. We included symptomatic patients aged 18–65 years without risk of severe disease, and positive antigen/PCR test for SARS-CoV-2. Patients received (<jats:italic>Bifidobacterium (B.) lactis</jats:italic> BI040, <jats:italic>B. longum</jats:italic> BL020, <jats:italic>Lactobacillus (L) rhamnosus</jats:italic> LR110, <jats:italic>L. casei</jats:italic> LC130, <jats:italic>L. acidophilus</jats:italic> LA120, 5 billion CFU total) or placebo 1 capsule a day for 28 days and recorded symptoms. Three months later patients completed Post-COVID-19 Questionnaire (PCQ-19). On days 0–5 and 28–35, blood was sampled for IgG to nucleocapsid protein (NCP) and receptor binding domain (RBD)/spike 1 (S1) protein. The primary outcome measure was a patient global symptom score on day 10 of observation. The difference between groups was assessed using the Mann–Whitney <jats:italic>U</jats:italic> test.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Results</jats:title>\n <jats:p>Seventy-three patients were assessed for clinical endpoints and 44 patients were evaluated for antibody production. At day 10, the median global symptom score (interquartile range) was lower in the probiotic group (0.0 (0.0–2.0) vs. 2.0 (1.0–5.0), <jats:italic>P</jats:italic> < 0.05). The probiotic group had a shorter duration of fatigue and anxiety after COVID -19 (<jats:italic>P</jats:italic> < 0.05) and a greater change in IgG concentration on RBD/S1 (225.9 vs. 105.6 binding antibody units/mL, <jats:italic>P</jats:italic> < 0.05).</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Conclusions</jats:title>\n <jats:p>Use of probiotics alleviates acute and post-disease symptoms, and improves humoral immune response to viral antigens.</jats:p>\n </jats:sec><jats:sec>\n <jats:title>Trial registration</jats:title>\n <jats:p>Registered at clinicaltrials.gov as NCT04907877, June 1, 2021.</jats:p>\n </jats:sec>",
"alternative-id": [
"816"
],
"article-number": "4",
"assertion": [
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Received",
"name": "received",
"order": 1,
"value": "10 July 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "Accepted",
"name": "accepted",
"order": 2,
"value": "22 December 2023"
},
{
"group": {
"label": "Article History",
"name": "ArticleHistory"
},
"label": "First Online",
"name": "first_online",
"order": 3,
"value": "4 January 2024"
},
{
"group": {
"label": "Declarations",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 1
},
{
"group": {
"label": "Ethics approval and consent to participate",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 2,
"value": "Approvals were obtained from ethical committees at all study sites (Lviv Oblast Center for Disease Control and Prevention Ministry of Health of Ukraine, protocol #2 of 20 Apr 2021; Lviv Municipal Non-Profit Enterprise Third City Clinical Hospital, protocol #1 of 03 Jun 2021; Hemo Medica Clinic, protocol #2 of 09 Jun 2021; Vinnytsia Municipal Non-Profit Enterprise Center of Primary Health Care №2, protocol #24 of 18 Jan 2022; Chernivtsi Municipal Non-Profit Enterprise City Polyclinic №1, protocol #2 of 24 Jan 2022.The study was conducted in accordance with the principles of the Declaration of Helsinki and the privacy rights of patients were respected in compliance with Good Clinical Practices throughout the study. Before enrollment in the study, eligible participants had protocol-specific health insurance and were familiarized with the study procedures. A signed informed consent was obtained from all patients. This study was registered at ClinicalTrials.gov on July 01, 2021 before the first patient signed the informed consent form (identifier: NCT04907877)."
},
{
"group": {
"label": "Consent for publication",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 3,
"value": "Not applicable."
},
{
"group": {
"label": "Competing interests",
"name": "EthicsHeading"
},
"name": "Ethics",
"order": 4,
"value": "POK, IHP, LPS, ZРH, OSL, NRH received investigator fees; SVG received study management fee from Nordic Biotic Sp. z o.o. (Warsaw, Poland). NOI, OIM, ZLS reported no competing interests."
}
],
"author": [
{
"affiliation": [],
"family": "Kolesnyk",
"given": "Pavlo O.",
"sequence": "first"
},
{
"affiliation": [],
"family": "Paliy",
"given": "Iryna H.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Sydorchuk",
"given": "Larysa P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Hoda",
"given": "Zoriana P.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Ivanchenko",
"given": "Nataliya O.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lych",
"given": "Oksana S.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Huley",
"given": "Natalia R.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Matsyura",
"given": "Oksana I.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Slyuzar",
"given": "Zoryana L.",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Gerasymov",
"given": "Sergiy V.",
"sequence": "additional"
}
],
"container-title": "BMC Nutrition",
"container-title-short": "BMC Nutr",
"content-domain": {
"crossmark-restriction": false,
"domain": [
"link.springer.com"
]
},
"created": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T15:05:48Z",
"timestamp": 1704380748000
},
"deposited": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T15:10:19Z",
"timestamp": 1704381019000
},
"funder": [
{
"award": [
"# DT0003A-COV",
"# DT0003A-COV",
"# DT0003A-COV",
"# DT0003A-COV",
"# DT0003A-COV",
"# DT0003A-COV",
"# DT0003A-COV"
],
"name": "Nordic Biotic Sp. z o.o."
}
],
"indexed": {
"date-parts": [
[
2024,
1,
5
]
],
"date-time": "2024-01-05T00:15:48Z",
"timestamp": 1704413748419
},
"is-referenced-by-count": 0,
"issue": "1",
"issued": {
"date-parts": [
[
2024,
1,
4
]
]
},
"journal-issue": {
"issue": "1",
"published-online": {
"date-parts": [
[
2024,
12
]
]
}
},
"language": "en",
"license": [
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "tdm",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T00:00:00Z",
"timestamp": 1704326400000
}
},
{
"URL": "https://creativecommons.org/licenses/by/4.0",
"content-version": "vor",
"delay-in-days": 0,
"start": {
"date-parts": [
[
2024,
1,
4
]
],
"date-time": "2024-01-04T00:00:00Z",
"timestamp": 1704326400000
}
}
],
"link": [
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40795-023-00816-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/article/10.1186/s40795-023-00816-8/fulltext.html",
"content-type": "text/html",
"content-version": "vor",
"intended-application": "text-mining"
},
{
"URL": "https://link.springer.com/content/pdf/10.1186/s40795-023-00816-8.pdf",
"content-type": "application/pdf",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "297",
"original-title": [],
"prefix": "10.1186",
"published": {
"date-parts": [
[
2024,
1,
4
]
]
},
"published-online": {
"date-parts": [
[
2024,
1,
4
]
]
},
"publisher": "Springer Science and Business Media LLC",
"reference": [
{
"DOI": "10.1016/j.eclinm.2021.100986",
"author": "X Jin",
"doi-asserted-by": "publisher",
"first-page": "100986",
"journal-title": "EClinicalMedicine",
"key": "816_CR1",
"unstructured": "Jin X, Ren J, Li R, Gao Y, et al. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine. 2021;37:100986. https://doi.org/10.1016/j.eclinm.2021.100986.",
"volume": "37",
"year": "2021"
},
{
"DOI": "10.1016/s0149-2918(01)80137-5",
"author": "AM Fendrick",
"doi-asserted-by": "publisher",
"first-page": "1683",
"issue": "10",
"journal-title": "Clin Ther",
"key": "816_CR2",
"unstructured": "Fendrick AM, Saint S, Brook I, Jacobs MR, et al. Diagnosis and treatment of upper respiratory tract infections in the primary care setting. Clin Ther. 2001;23(10):1683–706. https://doi.org/10.1016/s0149-2918(01)80137-5.",
"volume": "23",
"year": "2001"
},
{
"DOI": "10.1001/jamainternmed.2018.2621",
"author": "M Incze",
"doi-asserted-by": "publisher",
"first-page": "1288",
"issue": "9",
"journal-title": "JAMA Intern Med",
"key": "816_CR3",
"unstructured": "Incze M, Grady D, Gupta A. I have a cold-what do i need to know? JAMA Intern Med. 2018;178(9):1288. https://doi.org/10.1001/jamainternmed.2018.2621.",
"volume": "178",
"year": "2018"
},
{
"key": "816_CR4",
"unstructured": "COVID-19 Treatment Guidelines Panel. Coronavirus disease 2019 (COVID-19) treatment guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov. Accessed 10 Oct 2023."
},
{
"DOI": "10.14309/ajg.0000000000000620",
"author": "L Pan",
"doi-asserted-by": "publisher",
"first-page": "766",
"journal-title": "Am J Gastroenterol",
"key": "816_CR5",
"unstructured": "Pan L, Mu M, Yang P, Sun Y, et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115:766–73. https://doi.org/10.14309/ajg.0000000000000620.",
"volume": "115",
"year": "2020"
},
{
"DOI": "10.1136/gutjnl-2020-323020",
"author": "YK Yeoh",
"doi-asserted-by": "publisher",
"first-page": "1",
"journal-title": "Gut",
"key": "816_CR6",
"unstructured": "Yeoh YK, Zuo T, Lui GC, Zhang F, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut. 2021;0:1–9. https://doi.org/10.1136/gutjnl-2020-323020.",
"volume": "0",
"year": "2021"
},
{
"DOI": "10.1111/eci.13276",
"author": "W Smyk",
"doi-asserted-by": "publisher",
"first-page": "e13276",
"issue": "9",
"journal-title": "Eur J Clin Invest",
"key": "816_CR7",
"unstructured": "Smyk W, Janik MK, Portincasa P, Milkiewicz P, et al. COVID-19: focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Invest. 2020;50(9):e13276. https://doi.org/10.1111/eci.13276.",
"volume": "50",
"year": "2020"
},
{
"DOI": "10.3389/fmicb.2016.01220",
"author": "C Westermann",
"doi-asserted-by": "publisher",
"first-page": "1220",
"journal-title": "Front Microbiol",
"key": "816_CR8",
"unstructured": "Westermann C, Gleinser M, Corr SC, Riedel CU. A critical evaluation of bifidobacterial adhesion to the host tissue. Front Microbiol. 2016;7:1220. https://doi.org/10.3389/fmicb.2016.01220.",
"volume": "7",
"year": "2016"
},
{
"DOI": "10.3390/nu3050613",
"author": "ML Van Tassell",
"doi-asserted-by": "publisher",
"first-page": "613",
"issue": "5",
"journal-title": "Nutrients",
"key": "816_CR9",
"unstructured": "Van Tassell ML, Miller MJ. Lactobacillus adhesion to mucus. Nutrients. 2011;3(5):613–36. https://doi.org/10.3390/nu3050613.",
"volume": "3",
"year": "2011"
},
{
"DOI": "10.1038/s41538-020-00078-9",
"author": "AN Olaimat",
"doi-asserted-by": "publisher",
"first-page": "17",
"journal-title": "NPJ Sci Food",
"key": "816_CR10",
"unstructured": "Olaimat AN, Aolymat I, Al-Holy M, Ayyash M, et al. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci Food. 2020;4:17. https://doi.org/10.1038/s41538-020-00078-9.",
"volume": "4",
"year": "2020"
},
{
"DOI": "10.1016/j.cell.2017.01.022",
"author": "N Geva-Zatorsky",
"doi-asserted-by": "publisher",
"first-page": "928",
"issue": "5",
"journal-title": "Cell",
"key": "816_CR11",
"unstructured": "Geva-Zatorsky N, Sefik E, Kua L, Pasman L, et al. Mining the human gut microbiota for immunomodulatory organisms. Cell. 2017;168(5):928-43.e11. https://doi.org/10.1016/j.cell.2017.01.022.",
"volume": "168",
"year": "2017"
},
{
"DOI": "10.3389/fmicb.2020.01302",
"author": "Y He",
"doi-asserted-by": "publisher",
"first-page": "1302",
"journal-title": "Front Microbiol",
"key": "816_CR12",
"unstructured": "He Y, Wang J, Li F, Shi Y. Main clinical features of COVID-19 and potential prognostic and therapeutic value of the microbiota in SARS-CoV-2 infections. Front Microbiol. 2020;11:1302. https://doi.org/10.3389/fmicb.2020.01302.",
"volume": "11",
"year": "2020"
},
{
"DOI": "10.1007/s00705-012-1543-0",
"author": "W Chai",
"doi-asserted-by": "publisher",
"first-page": "799",
"journal-title": "Arch Virol",
"key": "816_CR13",
"unstructured": "Chai W, Burwinkel M, Wang Z, et al. Antiviral effects of a probiotic Enterococcusfaecium strain against transmissible gastroenteritis coronavirus. Arch Virol. 2013;158:799–807. https://doi.org/10.1007/s00705-012-1543-0.",
"volume": "158",
"year": "2013"
},
{
"DOI": "10.1080/1040841X.2016.1176988",
"author": "Y He",
"doi-asserted-by": "publisher",
"first-page": "81",
"journal-title": "Crit Rev Microbiol",
"key": "816_CR14",
"unstructured": "He Y, Wen Q, Yao F, Xu D, et al. Gut-lung axis: the microbial contributions and clinical implications. Crit Rev Microbiol. 2017;43:81–95. https://doi.org/10.1080/1040841X.2016.1176988.",
"volume": "43",
"year": "2017"
},
{
"DOI": "10.3390/microorganisms10112185",
"author": "F Raya Tonetti",
"doi-asserted-by": "publisher",
"first-page": "2185",
"issue": "11",
"journal-title": "Microorganisms",
"key": "816_CR15",
"unstructured": "Raya Tonetti F, Clua P, Fukuyama K, Marcial G, et al. The ability of postimmunobiotics from L.rhamnosus CRL1505 to protect against respiratory syncytial virus and pneumococcal super-infection is a strain-dependent characteristic. Microorganisms. 2022;10(11):2185. https://doi.org/10.3390/microorganisms10112185.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.1002/14651858.CD006895.pub4",
"author": "Y Zhao",
"doi-asserted-by": "publisher",
"first-page": "CD006895",
"issue": "8",
"journal-title": "Cochrane Database Syst Rev",
"key": "816_CR16",
"unstructured": "Zhao Y, Dong BR, Hao Q. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2022;8(8):CD006895. https://doi.org/10.1002/14651858.CD006895.pub4.",
"volume": "8",
"year": "2022"
},
{
"DOI": "10.1001/jama.2020.12603",
"author": "A Carfì",
"doi-asserted-by": "publisher",
"first-page": "603",
"issue": "6",
"journal-title": "JAMA",
"key": "816_CR17",
"unstructured": "Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603–5. https://doi.org/10.1001/jama.2020.12603.",
"volume": "324",
"year": "2020"
},
{
"key": "816_CR18",
"unstructured": "World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus. Geneva: WHO; 2021. https://iris.who.int/bitstream/handle/10665/345824/WHO-2019-nCoV-Post-COVID-19-condition-Clinical-case-definition-2021.1-eng.pdf. Accessed 17 Dec 2021."
},
{
"DOI": "10.1183/13993003.01494-2020",
"author": "FA Klok",
"doi-asserted-by": "publisher",
"first-page": "2001494",
"journal-title": "Eur Respir J",
"key": "816_CR19",
"unstructured": "Klok FA, Boon GJAM, Barco S, et al. The post-COVID-19 functional status scale: a tool to measure functional status over time after COVID-19. Eur Respir J. 2020;56:2001494. https://doi.org/10.1183/13993003.01494-2020.",
"volume": "56",
"year": "2020"
},
{
"key": "816_CR20",
"unstructured": "WHO R&D Blueprint Novel Coronavirus COVID-19 therapeutic trial synopsis. World Health Organization; 2020. https://www.who.int/blueprint/priority-diseases/key-action/COVID-19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf. Accessed 7 Dec 2020."
},
{
"DOI": "10.1016/S0140-6736(20)30183-5",
"author": "C Huang",
"doi-asserted-by": "publisher",
"first-page": "497",
"journal-title": "Lancet",
"key": "816_CR21",
"unstructured": "Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. https://doi.org/10.1016/S0140-6736(20)30183-5.",
"volume": "395",
"year": "2020"
},
{
"DOI": "10.1001/jama.2020.1585",
"author": "D Wang",
"doi-asserted-by": "publisher",
"first-page": "1061",
"issue": "11",
"journal-title": "China JAMA",
"key": "816_CR22",
"unstructured": "Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323(11):1061–9. https://doi.org/10.1001/jama.2020.1585.",
"volume": "323",
"year": "2020"
},
{
"DOI": "10.1016/S0140-6736(20)30566-3",
"doi-asserted-by": "publisher",
"key": "816_CR23",
"unstructured": "Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. 2020;395:1054–62.https://doi.org/10.1016/S0140-6736(20)30566-3."
},
{
"DOI": "10.1016/S2213-2600(20)30079-5",
"author": "X Yang",
"doi-asserted-by": "publisher",
"first-page": "475",
"issue": "5",
"journal-title": "Lancet Respir Med",
"key": "816_CR24",
"unstructured": "Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. https://doi.org/10.1016/S2213-2600(20)30079-5.",
"volume": "8",
"year": "2020"
},
{
"DOI": "10.2807/1560-7917.ES.2021.26.24.2100509",
"doi-asserted-by": "publisher",
"key": "816_CR25",
"unstructured": "Campbell F, Archer B, Laurenson-Schafer H, Jinnai Y, et al. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 26(24): 2100509. https://doi.org/10.2807/1560-7917.ES.2021.26.24.2100509."
},
{
"key": "816_CR26",
"unstructured": "State institution “Lviv oblast center for diseases control and prevention of the Ministry of Health of Ukraine”. Coronavirus in Lviv State. https://www.cdc.lviv.ua/en/post/coronavirus-in-lviv-oblast-3. Accessed 31 Mar 2023."
},
{
"DOI": "10.1016/S0140-6736(22)00462-7",
"author": "T Nyberg",
"doi-asserted-by": "publisher",
"first-page": "1303",
"issue": "10332",
"journal-title": "Lancet",
"key": "816_CR27",
"unstructured": "Nyberg T, Ferguson NM, Nash SG, Webster HH, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–12. https://doi.org/10.1016/S0140-6736(22)00462-7.",
"volume": "399",
"year": "2022"
},
{
"DOI": "10.21037/apm-21-3373",
"author": "H Wang",
"doi-asserted-by": "publisher",
"first-page": "12374",
"journal-title": "Ann Palliat Med",
"key": "816_CR28",
"unstructured": "Wang H, Wang Y, Lu C, Qiu L, et al. The efficacy of probiotics in patients with severe COVID-19. Ann Palliat Med. 2021;10:12374–80. https://doi.org/10.21037/apm-21-3373.",
"volume": "10",
"year": "2021"
},
{
"DOI": "10.1016/j.medmic.2022.100061",
"author": "V Navarro-Lopez",
"doi-asserted-by": "publisher",
"first-page": "100061",
"journal-title": "Med Microecol",
"key": "816_CR29",
"unstructured": "Navarro-Lopez V, Hernandez-Belmonte A, Perez SM, Ayo-Gonzalez M, et al. Oral intake of Kluyveromycesmarxianus B0399 plus Lactobacillusrhamnosus CECT 30579 to mitigate symptoms in COVID-19 patients: a randomized open label clinical trial. Med Microecol. 2022;14:100061. https://doi.org/10.1016/j.medmic.2022.100061.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1080/19490976.2021.2018899",
"author": "P Gutiérrez-Castrellón",
"doi-asserted-by": "publisher",
"first-page": "2018899",
"issue": "1",
"journal-title": "Gut Microbes",
"key": "816_CR30",
"unstructured": "Gutiérrez-Castrellón P, Gandara-Martí T, Abreu Y, Abreu AT, Nieto-Rufino CD, et al. Probiotic improves symptomatic and viral clearance in Covid-19 outpatients: a randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes. 2022;14(1):2018899. https://doi.org/10.1080/19490976.2021.2018899.",
"volume": "14",
"year": "2022"
},
{
"DOI": "10.1177/17562848211035670",
"author": "L Zhang",
"doi-asserted-by": "publisher",
"first-page": "1088218646",
"journal-title": "Therap Adv Gastroenterol",
"key": "816_CR31",
"unstructured": "Zhang L, Han H, Li X, Chen C, et al. Probiotics use is associated with improved clinical outcomes among hospitalized patients with COVID-19. Therap Adv Gastroenterol. 2021;14:1088218646. https://doi.org/10.1177/17562848211035670.",
"volume": "14",
"year": "2021"
},
{
"DOI": "10.1128/spectrum.01682-22",
"author": "I de Boeck",
"doi-asserted-by": "publisher",
"first-page": "e0168222",
"issue": "5",
"journal-title": "Microbiol Spectr",
"key": "816_CR32",
"unstructured": "de Boeck I, Cauwenberghs E, Spacova I, Gehrmann T, et al. Randomized, double-blind, placebo-controlled trial of a throat spray with selected lactobacilli in COVID-19 outpatients. Microbiol Spectr. 2022;10(5):e0168222. https://doi.org/10.1128/spectrum.01682-22.",
"volume": "10",
"year": "2022"
},
{
"DOI": "10.3389/fmed.2020.00389",
"author": "G d’Ettorre",
"doi-asserted-by": "publisher",
"first-page": "389",
"journal-title": "Front Med (Lausanne)",
"key": "816_CR33",
"unstructured": "d’Ettorre G, Ceccarelli G, Marazzato M, Campagna G, et al. Challenges in the management of SARS-CoV2 infection: the role of oral bacteriotherapy as complementary therapeutic strategy to avoid the progression of COVID-19. Front Med (Lausanne). 2020;7:389. https://doi.org/10.3389/fmed.2020.00389.",
"volume": "7",
"year": "2020"
},
{
"DOI": "10.1101/2021.04.18.21255699",
"doi-asserted-by": "publisher",
"key": "816_CR34",
"unstructured": "Endam LM, Tremblay C, Filali A, Desrosiers MY. Intranasal application of Lactococcuslactis W 136 bacteria early in SARS-Cov-2 infection may have a beneficial immunomodulatory effect: a proof-of-concept study. medRxiv. 2021.https://doi.org/10.1101/2021.04.18.21255699"
},
{
"DOI": "10.3390/nu15071736",
"author": "S Reino-Gelardo",
"doi-asserted-by": "publisher",
"first-page": "1736",
"issue": "7",
"journal-title": "Nutrients",
"key": "816_CR35",
"unstructured": "Reino-Gelardo S, Palop-Cervera M, Aparisi-Valero N, Espinosa-San Miguel I, et al. Effect of an immune-boosting, antioxidant and anti-inflammatory food supplement in hospitalized COVID-19 patients: a prospective randomized pilot study. Nutrients. 2023;15(7):1736. https://doi.org/10.3390/nu15071736.",
"volume": "15",
"year": "2023"
},
{
"DOI": "10.3390/jcm11133758",
"author": "A Saviano",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "J Clin Med",
"key": "816_CR36",
"unstructured": "Saviano A, Potenza A, Siciliano V, Petruzziello C, et al. COVID-19 pneumonia and gut inflammation: the role of a mix of three probiotic strains in reducing inflammatory markers and need for oxygen support. J Clin Med. 2022;11:13. https://doi.org/10.3390/jcm11133758.",
"volume": "11",
"year": "2022"
},
{
"DOI": "10.1002/jmv.28463",
"author": "M Vaezi",
"doi-asserted-by": "publisher",
"journal-title": "J Med Virol",
"key": "816_CR37",
"unstructured": "Vaezi M, Ravanshad S, Rad MA, Zarrinfar H, Kabiri M. The effect of synbiotic adjunct therapy on clinical and paraclinical outcomes in hospitalized COVID-19 patients: a randomized placebo-controlled trial. J Med Virol. 2023. https://doi.org/10.1002/jmv.28463.",
"year": "2023"
},
{
"DOI": "10.1073/pnas.1019378108",
"author": "T Ichinohe",
"doi-asserted-by": "publisher",
"first-page": "5354",
"issue": "13",
"journal-title": "Proc Natl Acad Sci USA",
"key": "816_CR38",
"unstructured": "Ichinohe T, Pang IK, Kumamoto Y, Peaper DR, et al. Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA. 2011;108(13):5354–9. https://doi.org/10.1073/pnas.1019378108.",
"volume": "108",
"year": "2011"
},
{
"DOI": "10.1016/j.immuni.2018.04.022",
"author": "A Trompette",
"doi-asserted-by": "publisher",
"first-page": "992",
"issue": "5",
"journal-title": "Immunity",
"key": "816_CR39",
"unstructured": "Trompette A, Gollwitzer ES, Pattaroni C, Lopez-Mejia IC, et al. Dietary fiber confers protection against flu by shaping Ly6c- patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity. 2018;48(5):992-1005.e8. https://doi.org/10.1016/j.immuni.2018.04.022.",
"volume": "48",
"year": "2018"
},
{
"DOI": "10.1128/cdli.9.1.105-108.2002.31",
"author": "T Hori",
"doi-asserted-by": "publisher",
"first-page": "105",
"issue": "1",
"journal-title": "Clin Diagn Lab Immunol",
"key": "816_CR40",
"unstructured": "Hori T, Kiyoshima J, Shida K, Yasui H. Augmentation of cellular immunity and reduction of influenza virus titer in aged mice fed Lactobacilluscasei strain Shirota. Clin Diagn Lab Immunol. 2002;9(1):105–8. https://doi.org/10.1128/cdli.9.1.105-108.2002.31.",
"volume": "9",
"year": "2002"
},
{
"DOI": "10.1016/j.intimp.2009.04.015",
"author": "N Maeda",
"doi-asserted-by": "publisher",
"first-page": "1122",
"issue": "9",
"journal-title": "Int Immunopharmacol",
"key": "816_CR41",
"unstructured": "Maeda N, Nakamura R, Hirose Y, Murosaki S, et al. Oral administration of heat-killed Lactobacillusplantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int Immunopharmacol. 2009;9(9):1122–5. https://doi.org/10.1016/j.intimp.2009.04.015.",
"volume": "9",
"year": "2009"
},
{
"DOI": "10.1016/j.celrep.2019.05.105",
"author": "KC Bradley",
"doi-asserted-by": "publisher",
"first-page": "245",
"issue": "1",
"journal-title": "Cell Rep",
"key": "816_CR42",
"unstructured": "Bradley KC, Finsterbusch K, Schnepf D, Crotta S, et al. Microbiota-driven tonic interferon signals in lung stromal cells protect from influenza virus infection. Cell Rep. 2019;28(1):245-56.e4. https://doi.org/10.1016/j.celrep.2019.05.105.",
"volume": "28",
"year": "2019"
},
{
"DOI": "10.1007/s00394-015-1056-1",
"author": "K Shida",
"doi-asserted-by": "publisher",
"first-page": "45",
"issue": "1",
"journal-title": "Eur J Nutr",
"key": "816_CR43",
"unstructured": "Shida K, Sato T, Iizuka R, Hoshi R, et al. Daily intake of fermented milk with Lactobacilluscasei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur J Nutr. 2017;56(1):45–53. https://doi.org/10.1007/s00394-015-1056-1.",
"volume": "56",
"year": "2017"
},
{
"DOI": "10.5414/cpp43318",
"author": "P Winkler",
"doi-asserted-by": "publisher",
"first-page": "318",
"issue": "7",
"journal-title": "Int J Clin Pharmacol Ther",
"key": "816_CR44",
"unstructured": "Winkler P, de Vrese M, Laue Ch, Schrezenmeir J. Effect of a dietary supplement containing probiotic bacteria plus vitamins and minerals on common cold infections and cellular immune parameters. Int J Clin Pharmacol Ther. 2005;43(7):318–26. https://doi.org/10.5414/cpp43318.",
"volume": "43",
"year": "2005"
},
{
"DOI": "10.1016/j.synbio.2018.03.001",
"author": "H Zhang",
"doi-asserted-by": "publisher",
"first-page": "113",
"issue": "2",
"journal-title": "Synth Syst Biotechnol",
"key": "816_CR45",
"unstructured": "Zhang H, Yeh C, Jin Z, Ding L, et al. Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol. 2018;3(2):113–20. https://doi.org/10.1016/j.synbio.2018.03.001.",
"volume": "3",
"year": "2018"
},
{
"DOI": "10.1038/s41586-020-2571-7",
"author": "L Liu",
"doi-asserted-by": "publisher",
"first-page": "450",
"issue": "7821",
"journal-title": "Nature",
"key": "816_CR46",
"unstructured": "Liu L, Wang P, Nair MS, Yu J, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584(7821):450–6. https://doi.org/10.1038/s41586-020-2571-7.",
"volume": "584",
"year": "2020"
},
{
"author": "DK Flaherty",
"first-page": "23",
"key": "816_CR47",
"unstructured": "Flaherty DK. Immunogenicity and antigenicity. In: Immunology for pharmacy. Amsterdam: Elsevier; 2012. p. 23–9.",
"volume-title": "Immunology for pharmacy",
"year": "2012"
},
{
"DOI": "10.3390/biom11121812",
"author": "M Maffei",
"doi-asserted-by": "publisher",
"first-page": "1812",
"issue": "12",
"journal-title": "Biomolecules",
"key": "816_CR48",
"unstructured": "Maffei M, Montemiglio LC, Vitagliano G, Fedele L, et al. The nuts and bolts of SARS-CoV-2 spike receptor-binding domain heterologous expression. Biomolecules. 2021;11(12):1812. https://doi.org/10.3390/biom11121812.",
"volume": "11",
"year": "2021"
},
{
"DOI": "10.1038/s41586-021-03237-4",
"author": "BA Johnson",
"doi-asserted-by": "publisher",
"first-page": "293",
"issue": "7849",
"journal-title": "Nature",
"key": "816_CR49",
"unstructured": "Johnson BA, Xie X, Bailey AL, Kalveram B, et al. Loss of furin cleavage site attenuates SARS-CoV-2 pathogenesis. Nature. 2021;591(7849):293–9. https://doi.org/10.1038/s41586-021-03237-4.",
"volume": "591",
"year": "2021"
},
{
"DOI": "10.1016/j.bbrc.2020.04.136",
"author": "W Zeng",
"doi-asserted-by": "publisher",
"first-page": "618",
"issue": "3",
"journal-title": "Biochem Biophys Res Commun",
"key": "816_CR50",
"unstructured": "Zeng W, Liu G, Ma H, Zhao D, et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527(3):618–23. https://doi.org/10.1016/j.bbrc.2020.04.136.",
"volume": "527",
"year": "2020"
},
{
"DOI": "10.1126/science.1195568",
"author": "YK Lee",
"doi-asserted-by": "publisher",
"first-page": "1768",
"issue": "6012",
"journal-title": "Science",
"key": "816_CR51",
"unstructured": "Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330(6012):1768–73. https://doi.org/10.1126/science.1195568.",
"volume": "330",
"year": "2010"
},
{
"DOI": "10.4274/balkanmedj.galenos.2022.2021-8-131",
"author": "HÖ Dinç",
"doi-asserted-by": "publisher",
"first-page": "172",
"issue": "3",
"journal-title": "Balkan Med J",
"key": "816_CR52",
"unstructured": "Dinç HÖ, Demirci M, Özdemir YE, Sirekbasan S, et al. Anti-SARS-CoV-2 IgG and neutralizing antibody levels in patients with past COVID-19 infection: a longitudinal study. Balkan Med J. 2022;39(3):172–7. https://doi.org/10.4274/balkanmedj.galenos.2022.2021-8-131.",
"volume": "39",
"year": "2022"
},
{
"DOI": "10.1016/j.jns.2022.120162",
"author": "L Premraj",
"doi-asserted-by": "publisher",
"first-page": "120162",
"journal-title": "J Neurol Sci",
"key": "816_CR53",
"unstructured": "Premraj L, Kannapadi NV, Briggs J, Seal SM, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. https://doi.org/10.1016/j.jns.2022.120162.",
"volume": "434",
"year": "2022"
},
{
"DOI": "10.1016/j.neubiorev.2019.03.023",
"author": "RT Liu",
"doi-asserted-by": "publisher",
"first-page": "13",
"journal-title": "Neurosci Biobehav Rev",
"key": "816_CR54",
"unstructured": "Liu RT, Walsh RFL, Sheehan AE. Prebiotics and probiotics for depression and anxiety: a systematic review and meta-analysis of controlled clinical trials. Neurosci Biobehav Rev. 2019;102:13–23. https://doi.org/10.1016/j.neubiorev.2019.03.023.",
"volume": "102",
"year": "2019"
},
{
"DOI": "10.1016/j.neubiorev.2017.09.016",
"author": "C de Weerth",
"doi-asserted-by": "publisher",
"first-page": "458",
"journal-title": "Neurosci Biobehav Rev",
"key": "816_CR55",
"unstructured": "de Weerth C. Do bacteria shape our development? Crosstalk between intestinal microbiota and HPA axis. Neurosci Biobehav Rev. 2017;83:458–71.",
"volume": "83",
"year": "2017"
},
{
"author": "E Daliri",
"first-page": "1e4",
"journal-title": "J Probiotics Health",
"key": "816_CR56",
"unstructured": "Daliri E, Oh D, Lee B. Psychobiotics; a promise for neurodevelopmental therapy. J Probiotics Health. 2016;4:1e4.",
"volume": "4",
"year": "2016"
},
{
"DOI": "10.1016/j.chom.2015.04.011",
"author": "TR Sampson",
"doi-asserted-by": "publisher",
"first-page": "565",
"issue": "5",
"journal-title": "Cell Host Microbe",
"key": "816_CR57",
"unstructured": "Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17(5):565–76.",
"volume": "17",
"year": "2015"
},
{
"DOI": "10.1016/j.neuron.2019.02.008",
"author": "C Fülling",
"doi-asserted-by": "publisher",
"first-page": "998",
"issue": "6",
"journal-title": "Neuron",
"key": "816_CR58",
"unstructured": "Fülling C, Dinan TG, Cryan JF. Gut microbe to brain signaling: what happens in vagus. Neuron. 2019;101(6):998–1002.",
"volume": "101",
"year": "2019"
}
],
"reference-count": 58,
"references-count": 58,
"relation": {},
"resource": {
"primary": {
"URL": "https://bmcnutr.biomedcentral.com/articles/10.1186/s40795-023-00816-8"
}
},
"score": 1,
"short-title": [],
"source": "Crossref",
"subject": [
"Public Health, Environmental and Occupational Health",
"Nutrition and Dietetics",
"Endocrinology, Diabetes and Metabolism",
"Medicine (miscellaneous)"
],
"subtitle": [],
"title": "The role of nutritional support with probiotics in outpatients with symptomatic acute respiratory tract infections: a multicenter, randomized, double-blind, placebo-controlled dietary study",
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.1007/springer_crossmark_policy",
"volume": "10"
}