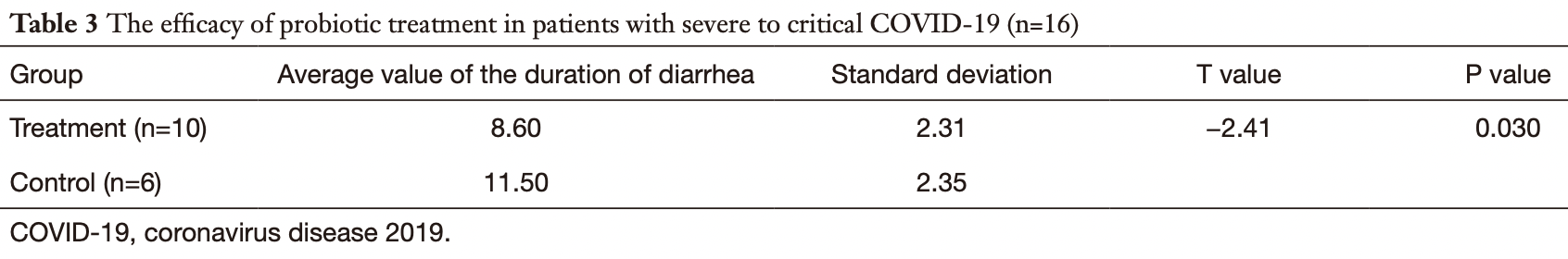
The efficacy of probiotics in patients with severe COVID-19
et al., Annals of Palliative Medicine, doi:10.21037/apm-21-3373, Dec 2021
Probiotics for COVID-19
20th treatment shown to reduce risk in
March 2021, now with p = 0.00000044 from 29 studies.
No treatment is 100% effective. Protocols
combine treatments.
6,400+ studies for
210+ treatments. c19early.org
|
Retrospective 156 COVID-19 patients in China, showing that diarrhea was significantly more common in severe/critical cases, and for severe/critical patients experiencing diarrhea, the duration of diarrhea was shorter with probiotic treatment. There was also significant improvements in inflammatory markers and time to PCR-. Details of the treatment and control groups are not supplied.
Probiotic efficacy depends on the specific strains used. Specific microbes may decrease or increase COVID-19 risk1.
Wang et al., 31 Dec 2021, retrospective, China, peer-reviewed, 8 authors.
The efficacy of probiotics in patients with severe COVID-19
Annals of Palliative Medicine, doi:10.21037/apm-21-3373
Background: To examine the incidence of diarrhea in severe and critical coronavirus disease 2019 (COVID-19) patients, and to observe the efficacy and prognosis of probiotic use in such patients. Methods: A retrospective study was conducted to investigate the symptoms and incidence of diarrhea in 156 cases of COVID-19 confirmed by the First Affiliated Hospital of Zhengzhou University and the Xinyang Fifth People's Hospital, China. A total of 58 cases of severe and critical COVID-19 were identified and divided into the treatment group or the control group. The control group was given standard treatment according to the Protocols for Diagnosis and Treatment of COVID-19: Prevention, Control, Diagnosis and Management. Patients in the treatment group were administered oral probiotics as well as the standard treatment. The 2 groups were compared in terms of nutritional status (serum albumin), improvement of diarrhea symptoms, changes in inflammatory condition [procalcitonin (PCT) and C-reactive protein (CRP)], the time taken to register a negative result for respiratory tract pathogens on the nucleic acid test, and changes to white blood cell and lymphocyte cell counts. Results: In this study cohort, diarrhea was detected in 15.38% (24/156) of COVID-19 patients. The incidence of diarrhea in patients with mild and moderate COVID-19 was approximately 8.16% (8/98), and the incidence of diarrhea in severe and critically ill patients was approximately 27.59% (16/58). In patients with severe and critical COVID-19, probiotic treatment obviously shortened the duration of diarrhea. Furthermore, compared with the control group, patients treated with probiotics showed a significantly reduced time to achieving a negative nucleic acid test and the inflammation indexes including PCT and CRP were significantly reduced (P<0.05). Conclusions: The incidence of diarrhea in severe and critically ill COVID-19 patients was significantly higher than that in patients with mild and moderate COVID-19. Probiotics may have a good supporting role in the treatment of patients with COVID-19 and its early application is recommended.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi. org/10.21037/apm-21-3373). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the First Affiliated Hospital of Zhengzhou University (No.: 2020-KY-056). Individual consent for this retrospective analysis was waived. Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the noncommercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
Center For, Control, Rapid investigation plan of clinical courses and key diagnosis and treatment equipment requirements for the patients of the 2019 Novel Coronavirus Pneumonia (COVID-19), Zhonghua Liu Xing Bing Xue Za Zhi
D'amico, Baumgart, Danese, Diarrhea During COVID-19 Infection: Pathogenesis, Epidemiology, Prevention, and Management, Clin Gastroenterol Hepatol
D'argenio, The role of the gut microbiome in the healthy adult status, Clin Chim Acta
Goto, Sagitani, Ashida, Anti-influenza virus effects of both live and non-live Lactobacillus acidophilus L-92 accompanied by the activation of innate immunity, Br J Nutr
Groves, Cuthbertson, James, Respiratory Disease following Viral Lung Infection Alters the Murine Gut Microbiota, Front Immunol
Harapan, Itoh, Yufika, Coronavirus disease 2019 (COVID-19): A literature review, J Infect Public Health
Harata, He, Hiruta, Intranasal administration of Lactobacillus rhamnosus GG protects mice from H1N1 influenza virus infection by regulating respiratory immune responses, Lett Appl Microbiol
Jiang, Deng, Zhang, Review of the Clinical Characteristics of Coronavirus Disease
Li, Lu, Li, The impact of COVID-19 on intestinal flora: A protocol for systematic review and meta analysis, Medicine
Liu, Kuo, Shih, COVID-19: The first documented coronavirus pandemic in history, Biomed J
Ma, Xia, Zhou, Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19, Clin Immunol
Marsland, Trompette, Gollwitzer, The Gut-Lung Axis in Respiratory Disease, Ann Am Thorac Soc
Mehta, Mcauley, Brown, COVID-19: consider cytokine storm syndromes and immunosuppression, Lancet
Mu, Yang, Zhu, Crosstalk Between The Immune Receptors and Gut Microbiota, Curr Protein Pept Sci
Shi, Wang, Cai, An overview of COVID-19, J Zhejiang Univ Sci B
Song, Liu, Shi, SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19, Gut
Van Nood, Speelman, Nieuwdorp, Fecal microbiota transplantation: facts and controversies, Curr Opin Gastroenterol
Vaninov, In the eye of the COVID-19 cytokine storm, Nat Rev Immunol
Wang, Li, Wei, Respiratory influenza virus infection induces intestinal immune injury via microbiotamediated Th17 cell-dependent inflammation, J Exp Med
Wang, Wang, Lu, Qiu, Song et al., The efficacy of probiotics in patients with severe COVID-19, Ann Palliat Med
DOI record:
{
"DOI": "10.21037/apm-21-3373",
"ISSN": [
"2224-5820",
"2224-5839"
],
"URL": "http://dx.doi.org/10.21037/apm-21-3373",
"author": [
{
"affiliation": [],
"family": "Wang",
"given": "Huaqi",
"sequence": "first"
},
{
"affiliation": [],
"family": "Wang",
"given": "Yunfei",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Lu",
"given": "Chunya",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Qiu",
"given": "Lingxiao",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Song",
"given": "Xiangjin",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Jia",
"given": "Hongxia",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Cui",
"given": "Dong",
"sequence": "additional"
},
{
"affiliation": [],
"family": "Zhang",
"given": "Guojun",
"sequence": "additional"
}
],
"container-title": [
"Annals of Palliative Medicine"
],
"content-domain": {
"crossmark-restriction": false,
"domain": [
"amegroups.com"
]
},
"created": {
"date-parts": [
[
2021,
12,
29
]
],
"date-time": "2021-12-29T02:11:15Z",
"timestamp": 1640743875000
},
"deposited": {
"date-parts": [
[
2021,
12,
30
]
],
"date-time": "2021-12-30T02:05:50Z",
"timestamp": 1640829950000
},
"indexed": {
"date-parts": [
[
2021,
12,
30
]
],
"date-time": "2021-12-30T06:00:20Z",
"timestamp": 1640844020734
},
"is-referenced-by-count": 0,
"issn-type": [
{
"type": "print",
"value": "2224-5820"
},
{
"type": "electronic",
"value": "2224-5839"
}
],
"issue": "12",
"issued": {
"date-parts": [
[
2021,
12
]
]
},
"journal-issue": {
"issue": "12",
"published-online": {
"date-parts": [
[
2021,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
}
},
"link": [
{
"URL": "https://apm.amegroups.com/article/download/86721/pdf",
"content-type": "unspecified",
"content-version": "vor",
"intended-application": "similarity-checking"
}
],
"member": "8611",
"original-title": [],
"page": "12374-12380",
"prefix": "10.21037",
"published": {
"date-parts": [
[
2021,
12
]
]
},
"published-online": {
"date-parts": [
[
2021,
12
]
]
},
"published-print": {
"date-parts": [
[
2021,
12
]
]
},
"publisher": "AME Publishing Company",
"reference-count": 0,
"references-count": 0,
"relation": {},
"score": 1,
"short-container-title": [
"Ann Palliat Med"
],
"short-title": [],
"source": "Crossref",
"subject": [
"Anesthesiology and Pain Medicine",
"Advanced and Specialized Nursing"
],
"subtitle": [],
"title": [
"The efficacy of probiotics in patients with severe COVID-19"
],
"type": "journal-article",
"update-policy": "http://dx.doi.org/10.21037/ame_crossmark_policy",
"volume": "10"
}
